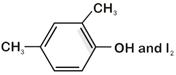
question_answer 1) An ester with molecular formula \[{{C}_{9}}{{H}_{10}}{{O}_{2}}\] was treated with excess of \[C{{H}_{3}}MgBr\] and the complex so formed was treated with \[{{H}_{2}}S{{O}_{4}}\] to give an olefin . Ozonolysis of gave a ketone with molecular formula \[{{C}_{8}}{{H}_{8}}O\] which shows +ve iodoform test. The structure of is: [AIPMT 1998]
A)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[{{H}_{3}}COC{{H}_{2}}CO{{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[p-{{H}_{3}}CO-{{C}_{6}}{{H}_{4}}-COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 2) Which one of the following esters cannot undergo Claisen self-condensation? [AIPMT 1998]
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{11}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 3) Aspirin is an acetylation product of: [AIPMT 1998]
A)
o-hydroxybenzoic acid
done
clear
B)
o-hydroxybenzene
done
clear
C)
m-hydroxybenzoic acid
done
clear
D)
p-dihydroxybenzene
done
clear
View Answer play_arrow
question_answer 4) Which one of the following compounds will react with \[NaHC{{O}_{3}}\] solution to give sodium salt and carbon dioxide? [AIPMT 1999]
A)
Acetic acid
done
clear
B)
n-hexanol
done
clear
C)
Phenol
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 5) Reduction by \[LiAl{{H}_{4}}\] of hydrolysed product of an ester gives: [AIPMT 2000]
A)
two acids
done
clear
B)
two aldehydes
done
clear
C)
one molecule of alcohol and another of carboxylic acid
done
clear
D)
two alcohols
done
clear
View Answer play_arrow
question_answer 6) Benzoic acid may be converted to ethyl benzoate by reaction with: [AIPMT 2000]
A)
sodium ethoxide
done
clear
B)
ethyl chloride
done
clear
C)
dry \[HCl-{{C}_{2}}{{H}_{s}}OH\]
done
clear
D)
ethanol
done
clear
View Answer play_arrow
question_answer 7)
In a set of the given reactions, acetic acid yielded a product C, \[C{{H}_{3}}COOH+PC{{l}_{5}}\to A\xrightarrow[Anh.\,AlC{{l}_{3}}]{{{C}_{6}}{{H}_{6}}}\]\[B\underset{Ether}{\mathop{\xrightarrow{{{C}_{2}}{{H}_{5}}MgBr}}}\,C\] product C would be : [AIPMT 2003]
A)
\[C{{H}_{3}}CH(OH){{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} {{C}_{2}}{{H}_{5}} \\ | \end{smallmatrix}}{\mathop{C(OH){{C}_{6}}{{H}_{5}}}}\,\]
done
clear
C)
\[C{{H}_{3}}CH(OH){{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}CO{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 8) The\[-OH\]group of an alcohol or the \[-COOH\]group of a carboxylic acid can be replaced by\[-Cl\]using: [AIPMT (S) 2004]
A)
phosphorus pentachloride
done
clear
B)
hypochlorous acid
done
clear
C)
chlorine
done
clear
D)
hydrochloric acid
done
clear
View Answer play_arrow
question_answer 9)
In a set of reactions, acetic acid yielded a product D. [AIPMT (S) 2005] \[C{{H}_{3}}COOH\xrightarrow[{}]{SOC{{l}_{2}}}A\underset{Anhyd.\,AlC{{l}_{3}}}{\mathop{\xrightarrow{Benzene}}}\,B\xrightarrow[{}]{HCN}C\xrightarrow[{}]{HOH}D\] The structure of D would be:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 10) Which one of the following compounds is most acidic? [AIPMT (S) 2005]
A)
\[Cl-C{{H}_{2}}-C{{H}_{2}}-OH\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 11) The enthalpy of combustion of H2, cyclohexene (C6H10) and cyclohexene (C6H12) are -241, -3800 and -3920 kJ per mol respectively. Heat of hydrogenation of cyclohexene is: [AIPMT (S) 2006]
A)
-121 kJ per mol
done
clear
B)
+121 kJ per mol
done
clear
C)
+242 kJ per mol
done
clear
D)
-242 kJ per mol
done
clear
View Answer play_arrow
question_answer 12) Self-condensation of two moles of ethyl acetate in presence of sodium ethoxide yields: [AIPMT (S) 2006]
A)
ethyl butyrate
done
clear
B)
acetoacetic ester
done
clear
C)
methyl acetoacetate
done
clear
D)
ethyl propionate
done
clear
View Answer play_arrow
A)
(ii) > (iv) > (iii) > (i)
done
clear
B)
(i) > (ii) > (iii) > (iv)
done
clear
C)
(iv) > (ii) > (i) > (iii)
done
clear
D)
(ii) > (iv) > (i) > (iii)
done
clear
View Answer play_arrow
question_answer 14) The relative reactivities of acyl compounds towards nucleophilic substitution are in the order of [AIPMT (S) 2008]
A)
Acyl chloride > Acid anhydride > Ester > Amide
done
clear
B)
Ester > Acyl chloride > Amide >Acid anhydride
done
clear
C)
Acid anhydride > Amide, > Ester > Acyl chloride
done
clear
D)
Acyl chloride > Ester > Acid anhydride > Amide
done
clear
View Answer play_arrow
question_answer 15) Propionic acid with \[B{{r}_{2}}-P\] yields a dibromo product. Its structure would be [AIPMT (S) 2009]
A)
\[\frac{d[B{{r}_{2}}]}{dt}=\frac{5}{3}\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
B)
\[\frac{d[B{{r}_{2}}]}{dt}=\frac{3}{5}\frac{d[B{{r}^{-}}]}{dt}\]
done
clear
C)
\[B{{F}_{3}},NO_{2}^{-},NH_{2}^{-}\]
done
clear
D)
\[{{H}_{2}}O,\]
done
clear
View Answer play_arrow
question_answer 16) Which one of the following compounds has the most acidic nature? [AIPMT (S) 2010]
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of these
done
clear
View Answer play_arrow
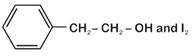
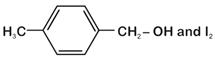
question_answer 17)
In a set of reactions, ethyl benzene yielded a product D. [AIPMT (S) 2010]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 18) Acetamide is treated with the following reagents separately. Which one of these would yield methyl amine? [AIPMT (S) 2010]
A)
\[NaOH-B{{r}_{2}}\]
done
clear
B)
Sodalime
done
clear
C)
Hot conc\[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[PC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 19) Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is [AIPMT (S) 2010]
A)
\[C{{H}_{3}}COOC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOCOC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COCl\]
done
clear
View Answer play_arrow
question_answer 20)
Among the following four compounds [AIPMT (M) 2010] A. Phenol B. Methyl phenol C. meta-nitrophenol D. para nitrophenol the acidity order is
A)
\[D>C>A>B\]
done
clear
B)
\[C>D>A>B\]
done
clear
C)
\[A>D>C>B\]
done
clear
D)
\[B>A>C>D\]
done
clear
View Answer play_arrow
question_answer 21)
Match the compounds given in list I with list II and select the suitable option using the code given bellows. [AIPMT (M) 2011] List I List II A. Benzaldehyde B. Phthalic anhydride C. Phenyl benzoate D. Methyl salicylate 1. Phenolphthalein 2. Benzoin condensation 3. Oil wintergreen 4. Fries rearrangement
Code
A)
A-4, B-1, C-3, D-2
done
clear
B)
A-4, B-2, C-3, D-1
done
clear
C)
A-2, B-3, C-4, D-1
done
clear
D)
A-2, B-1, C-4, D-3
done
clear
View Answer play_arrow
question_answer 22)
In the following sequence of reactions, [AIPMT (S) 2012] \[C{{H}_{3}}-Br\xrightarrow[{}]{KCN}A\xrightarrow[{}]{{{H}_{3}}{{O}^{+}}}B\xrightarrow[ether]{LiAl{{H}_{4}}}C\] the end product is
A)
acetone
done
clear
B)
methane
done
clear
C)
acetaldehyde
done
clear
D)
ethyl alcohol
done
clear
View Answer play_arrow
question_answer 23)
Consider the following reaction [AIPMT (M) 2012] The product 'A' is
A)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
View Answer play_arrow
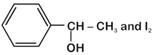
question_answer 24) Which of the following compounds will give a yellow precipitate with iodine and alkali? [AIPMT (M) 2012]
A)
Acetophenone
done
clear
B)
Methyl acetate
done
clear
C)
Acetamide
done
clear
D)
2-hydroxypropane
done
clear
View Answer play_arrow
question_answer 25) Reaction by which benzaldehyde cannot be prepared? [NEET 2013]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 26) Which one of the following esters gets hydrolysed most easily under alkaline conditions? [NEET 2015 (Re)]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 27)
The following reaction, [NEET 2015 (Re)]
A)
Friedel-Crafts reaction
done
clear
B)
Perkins reaction
done
clear
C)
Acetylation reaction
done
clear
D)
Schotten-Baumann reaction
done
clear
View Answer play_arrow
question_answer 28) A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{o}}_{\text{4}}}\]. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be [NEET - 2018]
A)
2.8
done
clear
B)
3.0
done
clear
C)
1.4
done
clear
D)
4.4
done
clear
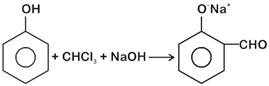
View Answer play_arrow
question_answer 29)
In the reaction The electrophile involved is [NEET - 2018]
A)
Dichloromethyl anion \[\left( \begin{align} & \oplus \\ & \text{CHC}{{\text{l}}_{\text{2}}} \\ \end{align} \right)\]
done
clear
B)
Formyl cation \[\left( \begin{align} & \oplus \\ & \text{CHO} \\ \end{align} \right)\]
done
clear
C)
Dichloromethyl cation \[\left( \begin{align} & \oplus \\ & \text{CHC}{{\text{l}}_{2}} \\ \end{align} \right)\]
done
clear
D)
Dichlorocarbene \[\text{(:CC}{{\text{l}}_{\text{2}}})\]
done
clear
View Answer play_arrow
question_answer 30) Compound \[\text{A,}{{\text{C}}_{\text{8}}}{{\text{H}}_{\text{10}}}\text{O,}\] is found to react with \[\text{NaOI}\] (produced by reacting Y with\[\text{NaOH}\]) and yields a yellow precipitate with characteristic smell. A and Y are respectively [NEET - 2018]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
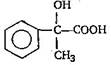
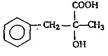
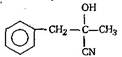
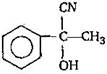
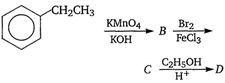 'D' would be
'D' would be 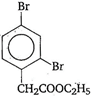
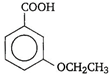
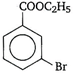
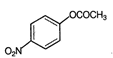
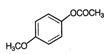
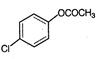
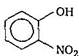
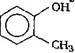
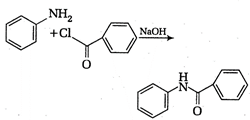 is known by the name
is known by the name