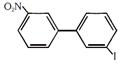
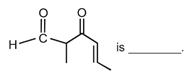
question_answer 1)
IUPAC name of the compounds [AIPMT 1998]
A)
trans-3 iodo- 4 chloro-3-pentene
done
clear
B)
cis-2-chloro-3-iodo-2-pentene
done
clear
C)
trans-2-chloro-3-iodo-2-pentene
done
clear
D)
cis-3-iodo-4-chloro-3-pentene
done
clear
View Answer play_arrow
question_answer 2) Which of the following compounds is not chiral? [AIPMT 1998]
A)
\[DC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHDCl\]
done
clear
C)
\[C{{H}_{3}}CHDC{{H}_{2}}Cl\]
done
clear
D)
\[C{{H}_{3}}CHClC{{H}_{2}}D\]
done
clear
View Answer play_arrow
question_answer 3) But-2-ene exhibits cu-trans isomerism due to: [AIPMT 2000]
A)
rotation around \[{{C}_{2}}{{C}_{3}}\] double bond
done
clear
B)
rotation around \[{{C}_{3}}{{C}_{4}}\] sigma bond
done
clear
C)
rotation around \[{{C}_{1}}{{C}_{2}}\] bond
done
clear
D)
restricted rotation around \[>C=C<\] bond
done
clear
View Answer play_arrow
question_answer 4) Among the following alkenes, [AIPMT 2000] 1-butene cis-2-butene trans-2-2butene I II III the decreasing order of stability is:
A)
II > I > III
done
clear
B)
III > II > I
done
clear
C)
III > I > II
done
clear
D)
I > II > III
done
clear
View Answer play_arrow
question_answer 5) The dihedral angle between two \[C-H\] bonds in the staggered conformation of ethane is: [AIPMT 2000]
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{180}^{o}}\]
done
clear
C)
\[{{0}^{o}}\]
done
clear
D)
\[{{120}^{o}}\]
done
clear
View Answer play_arrow
question_answer 6) The (R) - and (S) - enantiomers of an optically active compound differ in: [AIPMT 2000]
A)
their solubility in a chiral solvents
done
clear
B)
their reactivity with a chiral reagents
done
clear
C)
their optical rotation of plane polarised light
done
clear
D)
their melting points
done
clear
View Answer play_arrow
question_answer 7) The incorrect IUPAC name is: [AIPMT 2001]
A)
\[\underset{\text{2, methyl-3-butanone}}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
B)
\[\underset{\text{2, 3-dimethyl pentane}}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}-C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
C)
\[\underset{\text{4-methyl-2-pentyne}}{\mathop{C{{H}_{3}}-C\equiv CCH{{(C{{H}_{3}})}_{2}}}}\,\]
done
clear
D)
\[\underset{\text{2-bromo-3-chlorobutane}}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
View Answer play_arrow
question_answer 8) A compound of molecular formula \[{{C}_{7}}{{H}_{16}}\] shows optical isomerism, compound will be: [AIPMT 2001]
A)
2, 3-dimethyI pentane
done
clear
B)
2, 2-dimethyl butane
done
clear
C)
2-methyl hexane
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 9) IUPAC name of die following is: [AIPMT 2002] \[C{{H}_{2}}=\text{ }CHC{{H}_{2}}C{{H}_{2}}C\equiv CH\]
A)
1, 5-hexenyne
done
clear
B)
1 - hexene-5-yne
done
clear
C)
1-hexyne-5-ene
done
clear
D)
1, 5-hexynene
done
clear
View Answer play_arrow
question_answer 10) \[\overset{\begin{smallmatrix} \odot - \\ \centerdot \,\,\centerdot \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix} |\,\,\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] and \[C{{H}_{2}}=\underset{\begin{align} & | \\ & _{\centerdot }^{\centerdot }O\,_{\centerdot }^{\centerdot } \\ & \centerdot \,\,\centerdot \\ & \odot - \\ \end{align}}{\mathop{C}}\,-C{{H}_{3}}\] are: [AIPMT 2002]
A)
resonating structures
done
clear
B)
tautomers
done
clear
C)
geometrical isomers
done
clear
D)
optical isomers
done
clear
View Answer play_arrow
question_answer 11) Geometrical isomers are differ in [AIPMT 2002]
A)
position of functional group
done
clear
B)
position of atoms
done
clear
C)
spatial arrangement of atoms
done
clear
D)
length of carbon chain
done
clear
View Answer play_arrow
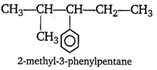
question_answer 12)
Name of the compound given below is: [AIPMT 2003]
A)
2, 3-diethylheptane
done
clear
B)
5-ethyl-6-methyloctane
done
clear
C)
4-ethyl-3-methyloctane
done
clear
D)
3-methyl-4-ethyloctane
done
clear
View Answer play_arrow
question_answer 13) Which of the following pairs of compounds are enantiomers? [AIPMT 2003]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 14)
The molecular formula of diphenyl methane
A)
6
done
clear
B)
4
done
clear
C)
8
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 15) Which one of the following pairs represents stereoisomerism? [AIPMT (S) 2005]
A)
Chain isomerism and rotational isomerism
done
clear
B)
Structural isomerism and geometric isomerism
done
clear
C)
Linkage isomerism and geometric isomerism
done
clear
D)
Optical isomerism and geomertric isomerism
done
clear
View Answer play_arrow
question_answer 16)
The chirality of the compound [AIPMT (S) 2005]
A)
R
done
clear
B)
S
done
clear
C)
Z
done
clear
D)
E
done
clear
View Answer play_arrow
question_answer 17) Names of some compounds are given. Which one is not correct in IUPAC system? [AIPMT (S) 2005]
A)
\[\underset{3-methyl-2\text{ }bu\tan ol}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
B)
\[\underset{4-methyl-2-pentyne}{\mathop{C{{H}_{3}}-C\equiv C}}\,-CH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[\underset{2-ethyl-3\text{ }methyl-but-1-ene}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ C{{H}_{2}} \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}}}\,\]
done
clear
D)
\[\underset{3-methyl-4\text{ }ehtyl\text{ }hep\tan e}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}C{{H}_{3}}}}\,\]
done
clear
View Answer play_arrow
question_answer 18) The best method for the separation of naphthalene and benzoic acid from their mixture is: [AIPMT (S) 2005]
A)
chromatography
done
clear
B)
crystallisation
done
clear
C)
distillation
done
clear
D)
sublimation
done
clear
View Answer play_arrow
question_answer 19) The general molecular formula, which represents the homologous series of alkanols is: [AIPMT (S) 2006]
A)
\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n}}O\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+1}}O\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n+2}}O\]
done
clear
View Answer play_arrow
question_answer 20) Which of the following is not chiral? [AIPMT (S) 2006]
A)
2-butanol
done
clear
B)
2, 3-dibromopentane
done
clear
C)
3-bromopentane
done
clear
D)
2-hydroxypropanoic acid
done
clear
View Answer play_arrow
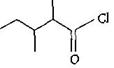
question_answer 21)
The IUPAC name of
A)
3, 4-dimethylpentanoyl chloride
done
clear
B)
1-chloro-1-oxo-2, 3-dimethylpentane
done
clear
C)
2-ethyl-3-methyIbutanoyi chloride
done
clear
D)
2, 3-dimethylpentanoyl chloride
done
clear
View Answer play_arrow
question_answer 22) If there is no rotation of plane polarized light by a compound in a specific solvent, thought to be chiral, it may mean that: [AIPMT (S) 2007]
A)
the compound is certainly a chiral
done
clear
B)
the compound is certainly meso
done
clear
C)
there is no compound in die solvent
done
clear
D)
the compound may be a racemic mixture
done
clear
View Answer play_arrow
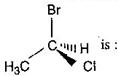
question_answer 23) \[C{{H}_{3}}-CHCl-C{{H}_{2}}-C{{H}_{3}}\] has a chiral centre. Which one of the following represents its R configuration? [AIPMT (S) 2007]
A)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ & {{H}_{3}}C-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-Cl \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,H \\ \end{align}\]
done
clear
B)
\[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ & H-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-C{{H}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,Cl \\ \end{align}\]
done
clear
C)
\[\begin{align} & \,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ & Cl-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-C{{H}_{3}} \\ & \,\,\,\,\,\,\,\,\,\,H \\ \end{align}\]
done
clear
D)
\[\begin{align} & \,\,\,\,\,\,\,\,\,C{{H}_{3}} \\ & H-\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-Cl \\ & \,\,\,\,\,\,\,\,\,{{C}_{2}}{{H}_{5}} \\ \end{align}\]
done
clear
View Answer play_arrow
question_answer 24) The IUPAC name of the compound having the formula \[CH\equiv C-CH=C{{H}_{2}}\] is [AIPMT (S) 2009]
A)
3-butene-1-yne
done
clear
B)
1-butyn-3-ene
done
clear
C)
but-1-yne-3-ene
done
clear
D)
1-butene-3-yne
done
clear
View Answer play_arrow
question_answer 25) Which of the following compounds will exhibit cis-trans (geometrical) isomerism? [AIPMT (S) 2009]
A)
2-butene
done
clear
B)
Butanol
done
clear
C)
2-butyne
done
clear
D)
2-butenol
done
clear
View Answer play_arrow
question_answer 26) Which/of the following conformers for ethylene glycol is most stable? [AIPMT (M) 2010]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 27) The IUPAC name of the compound\[C{{H}_{3}}CH=CHC\equiv CH\] [AIPMT (M) 2010]
A)
pent-4-yn-2-ene
done
clear
B)
pent-3-en-1-yne
done
clear
C)
pent-2-en-4-yne
done
clear
D)
pent-1-yn-3-ene
done
clear
View Answer play_arrow
question_answer 28)
The correct IUPAC name of the compound
A)
3-ethyl-4-ethenylheptane
done
clear
B)
3-ethyl-4-propylhex-5-ene
done
clear
C)
3-(1-ethyl propyl) hex-1-ene
done
clear
D)
4-ethyl-3-propylhex-1-ene
done
clear
View Answer play_arrow
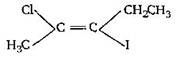
question_answer 29) The IUPAC name of the following compound [AIPMT (M) 2011]
A)
is trans-2-chloro-3-iodo-2-pentene
done
clear
B)
cis-3-iodo-4-chloro-3-pentene
done
clear
C)
rrans-3-iodo-4-chloro-3-pentene
done
clear
D)
cis-2-chloro-3-iodo-2-pentene
done
clear
View Answer play_arrow
question_answer 30) Which nomenclature is not according to IUPAC system? [AIPMT (S) 2012]
A)
\[\underset{1-bromo\,pro-2-ene}{\mathop{Br-C{{H}_{2}}-CH=C{{H}_{2}}}}\,\]
done
clear
B)
\[\underset{4-bromo-2,\,4-\dimethylhexane}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{3}}}}\,\]
done
clear
C)
done
clear
D)
\[\underset{5-oxohexanoic\,acid}{\mathop{C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-C{{H}_{2}}}}\,-C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 31) Which of the following acids does not exhibit optical isomerism? [AIPMT (S) 2012]
A)
Maleic acid
done
clear
B)
\[\alpha -\]amino acids
done
clear
C)
Lactic acid
done
clear
D)
Tartaric acid
done
clear
View Answer play_arrow
question_answer 32) Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy- 4-methylhex-3-en-5-ynoic acid is [NEET 2013]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 33) The structure of isobutyl group in an organic compound is [NEET 2013]
A)
done
clear
B)
\[C{{H}_{3}}-\underset{{}}{\mathop{CH}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-\]
done
clear
View Answer play_arrow
A)
I > II > III
done
clear
B)
III > II > I
done
clear
C)
II > l> III
done
clear
D)
II > III > I
done
clear
View Answer play_arrow
question_answer 35)
The enolic form of ethyl acetoacetate as below has [NEET 2015 ]
A)
18 sigma bonds and 2 pi-bonds
done
clear
B)
16 sigma bonds and 1 pi-bond
done
clear
C)
9 sigma bonds and 2 pi-bonds
done
clear
D)
9 sigma bonds and 1 pi-bond
done
clear
View Answer play_arrow
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and III
done
clear
D)
I, II and III
done
clear
View Answer play_arrow
question_answer 37)
The number of structural isomers possible from the molecular formula
A)
4
done
clear
B)
5
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 38)
Two possible stereo-structures of
A)
diastereomers
done
clear
B)
atropisomers
done
clear
C)
enantiomers
done
clear
D)
mesomers
done
clear
View Answer play_arrow
question_answer 39) The correct statement regarding the comparison of staggered and eclipsed conformation of ethane, is :- [NEET - 2016]
A)
The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain
done
clear
B)
The eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has no torsional strain
done
clear
C)
The eclipsed conformation of ethane is more stable than staggered conformation even through the eclipsed conformation has torsional strain
done
clear
D)
The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
done
clear
View Answer play_arrow
question_answer 40) Which of the following biphenyls is optically active?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
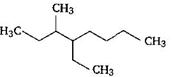
question_answer 41)
The IUPAC name of the compound [NEET-2017]
A)
3-keto-2-methylhex-5-enal
done
clear
B)
3-keto-2-methylhex-4-enal
done
clear
C)
5-formylhex-2-en-3-one
done
clear
D)
5-methyl-4-oxohex-2-en-5-al
done
clear
View Answer play_arrow
question_answer 42) With respect to the conformers of ethane, which of the following statements is true? [NEET-2017]
A)
Both bond angles and bond length remains same
done
clear
B)
Bond angle remains same but bond length changes
done
clear
C)
Bond angle changes but bond length remains
done
clear
D)
Both bond angle and bond length change
done
clear
View Answer play_arrow
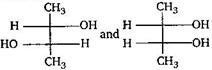

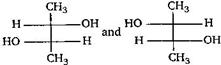
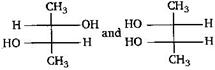
 is: [AIPMT (S) 2006]
is: [AIPMT (S) 2006]
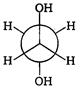
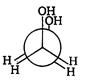
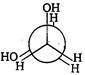
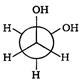
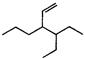 is [AIPMT (S) 2011]
is [AIPMT (S) 2011]
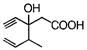
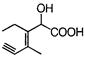
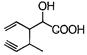
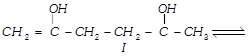
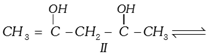
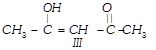
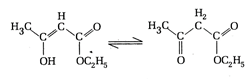
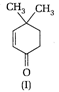
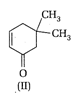
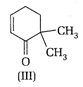 Which of the given compounds can exhibit tautomerism?
Which of the given compounds can exhibit tautomerism?