A) \[B{{I}_{3}}>BB{{r}_{3}}>B{{F}_{3}}>BC{{l}_{3}}\]
B) \[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\]
C) \[B{{F}_{3}}>BC{{l}_{3}}>BB{{r}_{3}}>B{{I}_{3}}\]
D) \[BC{{l}_{3}}>B{{F}_{3}}>B{{I}_{3}}>BB{{r}_{3}}\]
Correct Answer: B
Solution :
\[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\] This order can be easily explained on the basis of the tendency of the halogen atom to back donate its lone pair of electrons to the empty \[p-\]orbital of the boron atom through \[p\pi -p\pi \] bonding. Since the size of the vacant \[2p-\]orbital of \[B\] and the \[2p-\]orbital of F containing a lone pair of electrons are almost identical, therefore, the lone pair of electrons on \[F\] is donated towards the \[B\] atoms. Further due to back donation by three \[F\] atoms, \[B{{F}_{3}}\] can be represented as a resonance hybrid of the three structures.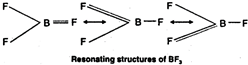 As a result of \[p\pi -p\pi \] back donation and resonance, the electron deficiency of \[B\] decreases and thus BF3 is the weakest Lewis acid. As the size of the halogen atom increases from \[Cl\] to \[I\], the extent of overlap between \[2p\] orbital of \[B\] and a bigger \[p-\]orbital of halogen (\[3p\] in \[Cl,\,\,4p\] in \[Br\] and \[5p\] in \[I\]) decreases and consequently the electron deficiency of \[B\] increases and thus the Lewis acid character increases accordingly from \[B{{F}_{3}}\] to \[B{{I}_{3}}\]. Thus, the relative acid strength of the boron trihalides follows the sequence: \[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\]
As a result of \[p\pi -p\pi \] back donation and resonance, the electron deficiency of \[B\] decreases and thus BF3 is the weakest Lewis acid. As the size of the halogen atom increases from \[Cl\] to \[I\], the extent of overlap between \[2p\] orbital of \[B\] and a bigger \[p-\]orbital of halogen (\[3p\] in \[Cl,\,\,4p\] in \[Br\] and \[5p\] in \[I\]) decreases and consequently the electron deficiency of \[B\] increases and thus the Lewis acid character increases accordingly from \[B{{F}_{3}}\] to \[B{{I}_{3}}\]. Thus, the relative acid strength of the boron trihalides follows the sequence: \[B{{I}_{3}}>BB{{r}_{3}}>BC{{l}_{3}}>B{{F}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
