A) \[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
B) \[{{W}_{2}}>{{W}_{3}}>{{W}_{1}}\]
C) \[{{W}_{1}}>{{W}_{2}}>{{W}_{3}}\]
D) \[{{W}_{1}}>{{W}_{3}}>{{W}_{2}}\]
Correct Answer: A
Solution :
The corresponding p-V graph (also called indicator diagram) in three different processes will be shown. Area under the graph gives the work done by the gas.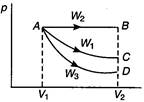 \[{{(Area)}_{2}}>{{(Area)}_{1}}>{{(Area)}_{3}}\] \[\therefore \] \[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
\[{{(Area)}_{2}}>{{(Area)}_{1}}>{{(Area)}_{3}}\] \[\therefore \] \[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
