A) ether is insoluble in water
B) methyl group is attached to oxygen in ether
C) the dipole moment of ethanol is greater
D) ethanol has H-bond
Correct Answer: D
Solution :
Due to the presence of intermolecular H-bonding the boiling point of ethyl alcohol is greater than dimethyl ether.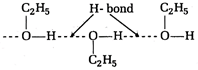
You need to login to perform this action.
You will be redirected in
3 sec
