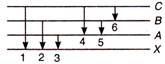
A) 1, 4, 5
B) 1, 2, 3, 4, 5
C) 2, 3, 5
D) 1, 2, 3
Correct Answer: D
Solution :
In absorption -spectrum, the electrons are excited from lower energy state to higher energy state. Thus absorption spectrum only 1, 2 and 3 lines will be obtained.You need to login to perform this action.
You will be redirected in
3 sec
