A) \[TiF_{6}^{2-}\]and\[C{{u}_{2}}C{{l}_{2}}\]
B) \[C{{u}_{2}}C{{l}_{2}}\]and\[NiCl_{4}^{2-}\]
C) \[TiF_{6}^{2-}\]and\[CoF_{6}^{3-}\]
D) \[CoF_{6}^{3-}\]and\[NiCl_{4}^{2-}\]
Correct Answer: A
Solution :
\[TiF_{6}^{2-},CoF_{6}^{3-},C{{u}_{2}}C{{l}_{2}}\] and\[NiCl_{4}^{2-}\] give \[T{{i}^{4+}},C{{o}^{3+}},C{{u}^{+}}\]and\[N{{i}^{2+}}\]ions respectively.| \[T{{i}^{4+}}=3{{d}^{0}},{{45}^{0}}\] | 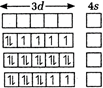 |
| \[C{{o}^{3+}}=3{{d}^{6}},{{45}^{0}}\] | |
| \[C{{u}^{+}}=3{{d}^{10}},{{45}^{0}}\] | |
| \[N{{i}^{2+}}=3{{d}^{8}},{{45}^{0}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
