A)
![]()
B)
![]()
C)
![]()
D)
![]()
Correct Answer: A
Solution :
The dipole moment of a molecule increases on increasing the eleetronegativity difference between two atoms. In formaldehyde the eleetronegativity difference between O and H is more hence, dipole moment of formaldehyde is maximum from the given compounds due to increase difference of charge.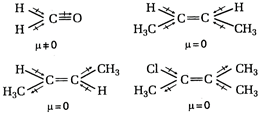
You need to login to perform this action.
You will be redirected in
3 sec
