A) \[C{{H}_{3}}CH(OH)Br\]
B) \[C{{H}_{3}}CH(OH)C{{H}_{3}}\]
C)
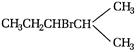
D) \[C{{H}_{3}}-CHOH-CHBr-C{{H}_{2}}OH\]
Correct Answer: B
Solution :
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]compound does not show optical isomerism due to the absence of asymmetrical carbon atom.You need to login to perform this action.
You will be redirected in
3 sec
