A) \[CI{{F}_{3}}\]
B) \[B{{F}_{3}}\]
C) \[Al{{F}_{3}}\]
D) \[N{{F}_{3}}\]
Correct Answer: A
Solution :
In\[CI{{F}_{3}}\]all bonds are not equal due to trigonal bypyramidal (\[s{{p}^{3}}d-\]hybridisation) geometry of\[Cl{{F}_{3}}\]molecule.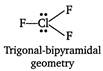 \[B{{F}_{3}}\]and\[Al{{F}_{3}}\]show trigonal symmetric structure due to \[s{{p}^{2}}-\]hybridisation.
\[B{{F}_{3}}\]and\[Al{{F}_{3}}\]show trigonal symmetric structure due to \[s{{p}^{2}}-\]hybridisation. You need to login to perform this action.
You will be redirected in
3 sec
