question_answer 1) F-or a triode amplification factor 20 and \[{{g}_{m}}=3\text{ }m\,\Omega ,\]If the resistance is\[3\times {{10}^{4}}\Omega ,\] the voltage gain will be
A)
16.36
done
clear
B)
28
done
clear
C)
78
done
clear
D)
108
done
clear
View Answer play_arrow
question_answer 2) Half-life of a radioactive element is T and its decay constant is \[\lambda \], then
A)
\[\lambda T=1\]
done
clear
B)
\[\lambda T=1/2\]
done
clear
C)
\[\lambda T={{\log }_{e}}2\]
done
clear
D)
\[\lambda =-{{\log }_{e}}2T\]
done
clear
View Answer play_arrow
question_answer 3) Internal resistance of cell does not depend upon the
A)
current taken by cell
done
clear
B)
distance between the electrode
done
clear
C)
concentration of electrolyte
done
clear
D)
emf of cell
done
clear
View Answer play_arrow
question_answer 4) Charge is quantized. This prove from
A)
experiment of Devisson- Jarmer
done
clear
B)
effect of Compton-scattering
done
clear
C)
Millikans oil drop experiment
done
clear
D)
Raman-scattering
done
clear
View Answer play_arrow
question_answer 5) The relative permittivity for diamagnetic material is
A)
\[-1<{{\mu }_{r}}<-10\]
done
clear
B)
\[1<{{\mu }_{r}}<10\]
done
clear
C)
\[-1<{{\mu }_{r}}>10\]
done
clear
D)
\[0<{{\mu }_{r}}>1\]
done
clear
View Answer play_arrow
question_answer 6) In an induction coil with resistance, the induced emf will be maximum when
A)
the switch is put on due to high resistance
done
clear
B)
the switch is put off due to high resistance
done
clear
C)
the switch is put on due to low resistance
done
clear
D)
the switch is put off due to low resistance
done
clear
View Answer play_arrow
question_answer 7) In stationary waves, the nodes have
A)
maximum energy
done
clear
B)
maximum change in pressure and density
done
clear
C)
maximum change in distortion
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 8) The ratio of diameter of 4th semi-frequency zone to 9th semi-frequency zone will be
A)
2/3
done
clear
B)
4/9
done
clear
C)
1/4
done
clear
D)
16/81
done
clear
View Answer play_arrow
question_answer 9) If the specific resistance and area of the potential meter wire are\[\rho \]and A respectively and the current / flows in the wire, then potential gradient will be
A)
\[I\rho /A\]
done
clear
B)
\[I/Ap\]
done
clear
C)
\[IA/\rho \]
done
clear
D)
\[I/A\rho \]
done
clear
View Answer play_arrow
question_answer 10) The de-Broglie wavelength related to a-particle accelerated by V volt will be
A)
\[\frac{0.287}{\sqrt{V}}\]\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[\frac{12.27}{\sqrt{V}}\]\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[\frac{4.34}{\sqrt{V}}\]\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\frac{0.202}{\sqrt{V}}\]\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 11) The peak value of AC current is 2\[\sqrt{2}\] A. Its apparent value will be
A)
1 A
done
clear
B)
2 A
done
clear
C)
4 A
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 12) The wavelength of first line of balmer series is 6563\[\overset{o}{\mathop{\text{A}}}\,\]. The wavelength of first line of lymen series will be
A)
1215.4\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
2500\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
7500\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
600\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 13) The nature of circuit is antiresonant of maximum frequency from resonant frequency will be
A)
resistance
done
clear
B)
capacitance
done
clear
C)
inductance
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 14) The reactance of inductance at\[{{10}^{4}}\]Hz is\[{{10}^{4}}\]\[\Omega \]. Its reactance at \[2\times {{10}^{4}}Hz\] will be
A)
\[{{10}^{4}}\Omega \]
done
clear
B)
\[2\times {{10}^{4}}\Omega \]
done
clear
C)
\[3\times {{10}^{4}}\Omega \]
done
clear
D)
\[4\times {{10}^{4}}\Omega \]
done
clear
View Answer play_arrow
question_answer 15) At NTP for the same volume of two gases of constant unit will be
A)
the total number of molecule
done
clear
B)
average kinetic energy
done
clear
C)
square mean root velocity
done
clear
D)
mean free path
done
clear
View Answer play_arrow
question_answer 16) At normal temperature for the diatomic gas, the number of degree of freedom is
A)
7
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 17) In capillery, meniscus of mercury is
A)
concave
done
clear
B)
convex
done
clear
C)
plane
done
clear
D)
circular
done
clear
View Answer play_arrow
question_answer 18) A ball is released from height h on the ground level. If the coefficient of restitution is e, then what height will after 2 times jump from ground?
A)
\[eh/2\]
done
clear
B)
\[2eh\]
done
clear
C)
\[eh\]
done
clear
D)
\[{{e}^{4}}h\]
done
clear
View Answer play_arrow
question_answer 19) The moment of inertia of a straight thin rod of mass M and length L about an axis perpendicular to its length and passing through its one end, is
A)
\[\frac{M{{L}^{2}}}{3}\]
done
clear
B)
\[\frac{M{{L}^{2}}}{12}\]
done
clear
C)
\[\frac{M{{L}^{2}}}{2}\]
done
clear
D)
\[\frac{M{{L}^{2}}}{13}\]
done
clear
View Answer play_arrow
question_answer 20) If the displacement of simple pendulum at any time is 0.02 m and acceleration is\[2\text{ }m/{{s}^{2}},\] then in this time angular velocity will be
A)
100 rad/s
done
clear
B)
10 rad/s
done
clear
C)
1 rad/s
done
clear
D)
0.1 rad/s
done
clear
View Answer play_arrow
question_answer 21) Which is constant, the earth revolving around the sun?
A)
Angular momentum
done
clear
B)
Linear momentum
done
clear
C)
Rotational kinetic energy
done
clear
D)
Kinetic energy
done
clear
View Answer play_arrow
question_answer 22)
Two springs of force constants k and 2k are connected to a mass M as shown below. The time period of oscillation of the mass M is
A)
\[2\pi \sqrt{\frac{3k}{M}}\]
done
clear
B)
\[2\pi \sqrt{\frac{k}{M}}\]
done
clear
C)
\[2\pi \sqrt{\frac{M}{2k}}\]
done
clear
D)
\[2\pi \sqrt{\frac{M}{3k}}\]
done
clear
View Answer play_arrow
question_answer 23) Two spheres each of mass M and radius R/2 are connected with a massless rod of length 2R as shown in the figure. What will be the moment of inertia of the system about an axis passing through the centre of one of the spheres and perpendicular to the rod?
A)
\[pV\]
done
clear
B)
\[2pV\]
done
clear
C)
\[3pV\]
done
clear
D)
\[4pV\]
done
clear
View Answer play_arrow
question_answer 24) In non- elastic collision
A)
momentum is conserved
done
clear
B)
energy is conserved
done
clear
C)
momentum and energy are conserved
done
clear
D)
momentum and energy are non conserved
done
clear
View Answer play_arrow
question_answer 25) In adiabatic process, the work done by system is 50 J, then
A)
the temperature of the system will be increase 50 J
done
clear
B)
the temperature of the system will be constant
done
clear
C)
the internal energy of the system will be increase 50 J
done
clear
D)
the internal energy of the system will be decrease 50 J
done
clear
View Answer play_arrow
question_answer 26)
An ideal monoatomic gas is taken round the cycle LMNO as shown in following diagram. The work done during the cycle is
A)
pV
done
clear
B)
2pV
done
clear
C)
3pV
done
clear
D)
4pV
done
clear
View Answer play_arrow
question_answer 27)
Water rise upto 5 cm in a straight capillary tube when its one end is dipped vertically in water. If it is bent according to the diagram, then the length of water column will be
A)
5 cm
done
clear
B)
less than 5 cm
done
clear
C)
more than 5 cm
done
clear
D)
5 cos \[\alpha \]
done
clear
View Answer play_arrow
question_answer 28) The requirement in phase relative source that those
A)
amplitudes are same
done
clear
B)
wavelengths are same
done
clear
C)
frequencies are same
done
clear
D)
first phase difference are same
done
clear
View Answer play_arrow
question_answer 29) In the Young's double slit experiment, intensities of black and bright fringes are 1 and 4 respectively, the ratio of amplitudes of sources will be
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
3 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 30) The primary reason of generation of Frounhofer line is
A)
reflection of radiations by chromosphere
done
clear
B)
absorption of radiation by chromosphere
done
clear
C)
emittion of radiation by chromosphere
done
clear
D)
transmission of radiation by chromospheres
done
clear
View Answer play_arrow
question_answer 31) The emf and current in an AC circuit are given by \[E=200\text{ }sin\text{ }314t\]volt and \[I=\,100\,\sin \,\left( 314+\frac{\pi }{3} \right)A,\] the coefficient of power will be
A)
1/2
done
clear
B)
1/4
done
clear
C)
1
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 32)
A pure resistance is attached as shown in figure. The phase difference between the emf and flowing current will be
A)
zero
done
clear
B)
\[\pi \]/2
done
clear
C)
\[-\pi \]/2
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 33) If inductance and resistance of chocke coil are\[{{X}_{L}}\] and R respectively, then
A)
\[{{X}_{L}}=r\]
done
clear
B)
\[{{X}_{L}}>>R\]
done
clear
C)
\[{{X}_{L}}<<R\]
done
clear
D)
\[{{X}_{L}}=\infty \]
done
clear
View Answer play_arrow
question_answer 34) The path difference, time difference and phase difference between two successive zones will be
A)
\[\frac{\lambda }{2},\frac{T}{2}and\pi \]
done
clear
B)
\[\lambda ,T\,and\,\pi \]
done
clear
C)
\[\frac{\lambda }{2},\frac{T}{2}and\,\frac{\pi }{2}\]
done
clear
D)
\[\frac{\lambda }{2},\frac{T}{2}and\,2\pi \]
done
clear
View Answer play_arrow
question_answer 35) The ozone layer is important because
A)
it prevents the cooling of each at night
done
clear
B)
it prevents the IR rays coming from the space
done
clear
C)
it prevents UV rays from the meteors coming from the space
done
clear
D)
it prevents the UV rays and micro rays the coming from the space
done
clear
View Answer play_arrow
question_answer 36) The number of zones for achieving the first minima on the screen due to circular gate will be
A)
4
done
clear
B)
6
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 37) In India for the use the fixed value of voltage and frequency of electricity are
A)
200 V, 60 Hz
done
clear
B)
220 V. 50 Hz
done
clear
C)
220 V, 60 Hz
done
clear
D)
220 V, 50 Hz
done
clear
View Answer play_arrow
question_answer 38) If resonant frequency is \[f\]and capacity become 4 times, then resonant frequency will be
A)
\[f/2\]
done
clear
B)
\[2f\]
done
clear
C)
\[f\]
done
clear
D)
\[f/4\]
done
clear
View Answer play_arrow
question_answer 39) The forbidden energy gap in the intrinsic semiconductor is
A)
0.1 eV
done
clear
B)
1 eV
done
clear
C)
5 eV
done
clear
D)
10 eV
done
clear
View Answer play_arrow
question_answer 40) How much kinetic energy will be gained by an \[\alpha \] -particle in going from a point at 70 V to another point at 50 V?
A)
40 eV
done
clear
B)
40 keV
done
clear
C)
40 MeV
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 41) The relation among magnetic induction B, magnetic field H and magnetic intensity \[I\]is
A)
\[B={{\mu }_{0}}H+I\]
done
clear
B)
\[B=~{{\mu }_{0}}I+H\]
done
clear
C)
\[B={{\mu }_{0}}(H+I)\]
done
clear
D)
\[B+{{\mu }_{0}}(H-I)\]
done
clear
View Answer play_arrow
question_answer 42) The velocity of sound in air is 333 m/s and the fundamental frequency of open pipe is 333 Hz. The length of pipe to produced second overtones will be
A)
0.5 m
done
clear
B)
1.0 m
done
clear
C)
1.5 m
done
clear
D)
2 m
done
clear
View Answer play_arrow
question_answer 43) If the length of sonometer wire is to be half, the value of resonance frequency will be
A)
three times
done
clear
B)
half
done
clear
C)
four times
done
clear
D)
twice
done
clear
View Answer play_arrow
question_answer 44) At what speed should a source of sound move so that stationary observer finds the apparent frequency equal to half of the original frequency?
A)
\[\frac{v}{2}\]
done
clear
B)
\[2v\]
done
clear
C)
\[\frac{v}{4}\]
done
clear
D)
\[v\]
done
clear
View Answer play_arrow
question_answer 45) The intensity of the X-rays in coolidge tube control
A)
by the current flowing in the filament
done
clear
B)
by potential between the cathode and anti- cathode
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 46) Which of the following is correct in terms of increasing work done for the same initial and final states?
A)
Adiabatic < Isothermal < Isobaric
done
clear
B)
Isobaric < Adiabatic < Isothermal
done
clear
C)
Adiabatic < Isobaric < Isothermal
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 47) A mica slit of thickness t and refractive index \[\mu \] is introduced in the ray from the first source\[{{S}_{1}}\]. By how much distance of fringes pattern will be displaced?
A)
\[\frac{d}{D}(\mu -1)t\]
done
clear
B)
\[\frac{D}{d}(\mu -1)t\]
done
clear
C)
\[\frac{d}{(\mu -1)D}\]
done
clear
D)
\[\frac{D}{d}(\mu -1)\]
done
clear
View Answer play_arrow
question_answer 48) The degree of air pressure in coolidge tube
A)
zero
done
clear
B)
\[{{10}^{-6}}\]mm (mercury)
done
clear
C)
\[{{10}^{-3}}\]mm (mercury)
done
clear
D)
1 mm (mercury)
done
clear
View Answer play_arrow
question_answer 49) The fundamental frequency of open pipe is n. The tube is immersed in water by placing in a vertical position so that its half part remain in the water. Now the fundamental frequency of air column will be
A)
\[\frac{n}{2}\]
done
clear
B)
\[\frac{2n}{4}\]
done
clear
C)
\[n\]
done
clear
D)
\[2n\]
done
clear
View Answer play_arrow
question_answer 50) The refractive index of water is 4/3 and that of glass is 5/3. What will be the critical angle for the ray of light entering water from the glass?
A)
\[{{\sin }^{-1}}\left( \frac{4}{5} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{5}{4} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{2}{1} \right)\]
done
clear
View Answer play_arrow
question_answer 51) A rocket emitting sound of frequency\[4\times {{10}^{7}}Hz\]is going away from the earth with a speed of 0.2 c, frequency of sound to be heard by the listener is
A)
\[4\times {{10}^{6}}Hz\]
done
clear
B)
\[3.3\times {{10}^{7}}Hz\]
done
clear
C)
\[3\times {{10}^{6}}Hz\]
done
clear
D)
\[5\times {{10}^{7}}Hz\]
done
clear
View Answer play_arrow
question_answer 52) The radius of fourth zone at observation point present at a distance of 4 m from the plane wave front of wavelength 6400\[\overset{o}{\mathop{\text{A}}}\,\] is
A)
\[32\times {{10}^{-4}}\]
done
clear
B)
\[32\times {{10}^{-4}}cm\]
done
clear
C)
\[32\times {{10}^{-4}}mm\]
done
clear
D)
\[16\times {{10}^{-2}}m\]
done
clear
View Answer play_arrow
question_answer 53) A normally incident ray reflected at angle of\[90{}^\circ \]. The value of critical angle is
A)
\[45{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[64{}^\circ \]
done
clear
D)
\[43.5{}^\circ \]
done
clear
View Answer play_arrow
question_answer 54) The produced rays in sonography are
A)
microwaves
done
clear
B)
infrared waves
done
clear
C)
sound waves
done
clear
D)
ultra sound
done
clear
View Answer play_arrow
question_answer 55) The ratio of secondary of primary turns of step up transformer is 4: 1. If a current of 4 A is applied to the primary, the induced current in secondary will be
A)
8 A
done
clear
B)
2 A
done
clear
C)
1 A
done
clear
D)
0.5 A
done
clear
View Answer play_arrow
question_answer 56) Two waves are represent by \[{{y}_{1}}=\alpha \sin \left( \omega t+\frac{\pi }{6} \right),{{y}_{2}}=a\cos \,\omega t,\]the resultant amplitude will be
A)
\[a\]
done
clear
B)
\[a\sqrt{2}\]
done
clear
C)
\[a\sqrt{3}\]
done
clear
D)
\[2a\]
done
clear
View Answer play_arrow
question_answer 57) The plate resistance of a triode is 15 k \[\Omega \] and voltage gain is 50. If load resistance is 100 k \[\Omega \] the amplification factor will be
A)
5
done
clear
B)
50
done
clear
C)
57.5
done
clear
D)
75.5
done
clear
View Answer play_arrow
question_answer 58) The relation between magnetic susceptibility\[\chi \] and temperature T of paramagnetic materials is
A)
\[\chi \]\[=C/T\]
done
clear
B)
\[\chi \]\[=CT\]
done
clear
C)
\[\chi \]\[=T/C\]
done
clear
D)
\[\chi \]\[=C{{T}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) At the plate voltage of 400 V and 200 V respectively the currents are\[{{i}_{{{p}_{1}}}}\]and\[{{i}_{{{p}_{2}}}}\]respectively. The ratio\[{{i}_{{{p}_{1}}}}/{{i}_{{{p}_{2}}}}\]will be
A)
\[1:2\]
done
clear
B)
\[2:1\]
done
clear
C)
\[1:2\sqrt{2}\]
done
clear
D)
\[2\sqrt{2}:1\]
done
clear
View Answer play_arrow
question_answer 60) At any instant the ratio of the amount of radioactive substances is 2:1. If their half lives be respectively 12 and 16 hours, then after two days, what will be the ratio of the substances?
A)
4 : 1
done
clear
B)
2 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 61) Moseley's law is
A)
\[v={{a}^{2}}(Z-b)\]
done
clear
B)
\[v=\frac{a}{(Z-b)}\]
done
clear
C)
\[v={{a}^{2}}{{(Z-b)}^{2}}\]
done
clear
D)
\[v=a\sqrt{(Z-b)}\]
done
clear
View Answer play_arrow
question_answer 62) Two bulbs of wattage 25 and 100 respectively are connected in series, which bulbs will fuse?
A)
25 W bulb
done
clear
B)
100 W bulb
done
clear
C)
Both [a] and [b]
done
clear
D)
None of them
done
clear
View Answer play_arrow
question_answer 63) The hardness of X-rays by coolidge tube depends upon
A)
filament current
done
clear
B)
air pressure in tube
done
clear
C)
material of target
done
clear
D)
potential between target and cathode
done
clear
View Answer play_arrow
question_answer 64)
In the circuit shown below, what will be the readings of the voltmeter and ammeter?
A)
800 V, 2A
done
clear
B)
300 V, 2A
done
clear
C)
220V, 2.2 A
done
clear
D)
100V, 2A
done
clear
View Answer play_arrow
question_answer 65) If the pressure of an ideal gas contained in a closed vessel is increased by 0.4%, the increases in temperature is\[1{}^\circ C\]. The initial temperature of the gas is
A)
\[25{}^\circ C\]
done
clear
B)
\[250{}^\circ C\]
done
clear
C)
250 K
done
clear
D)
\[2500{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 66) If a van der Waal's gas expands freely, then final temperature is
A)
less than the initial temperature
done
clear
B)
equal to the initial temperature
done
clear
C)
more than the initial temperature
done
clear
D)
less or more than the initial temperature depending on the nature of the gas
done
clear
View Answer play_arrow
question_answer 67) The required mechanical work to increase in per unit surface area of liquid is called surface tension of the liquid. When liquid is in
A)
isothermal condition
done
clear
B)
isobaric condition
done
clear
C)
isochoric condition
done
clear
D)
adiabatic condition
done
clear
View Answer play_arrow
question_answer 68) A person will get more quantity of matter in 1 kg-wt at
A)
poles
done
clear
B)
moon
done
clear
C)
equator
done
clear
D)
satellite
done
clear
View Answer play_arrow
question_answer 69) A sphere of mass m moving with a constant velocity u hits another stationary sphere of the same mass. Ife is the coefficient of restitution, then the ratio of the velocity of two spheres after collision will be
A)
\[\frac{1-e}{1+e}\]
done
clear
B)
\[\frac{e-1}{e+1}\]
done
clear
C)
\[\frac{1+e}{1-e}\]
done
clear
D)
\[\frac{e+1}{e-1}\]
done
clear
View Answer play_arrow
question_answer 70) The moment of inertia of anybody about an its axis is equal to moment of inertia about its perpendicular axis, body is
A)
disc
done
clear
B)
solid cylinder
done
clear
C)
ring
done
clear
D)
spherical shell
done
clear
View Answer play_arrow
question_answer 71) A clocks is based on oscillation of a spring and a clock P is based on pendulum motion. Both clocks run at the same rate on earth. On a planet having the same density as earth but twice the radius
A)
S will run faster than P
done
clear
B)
P will run faster than S
done
clear
C)
They will both run at the same rate as on the earth
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 72) The angular speed of minute needle in a watch
A)
\[\frac{\pi }{21600}rad/s\]
done
clear
B)
\[\frac{\pi }{12}rad/s\]
done
clear
C)
\[\frac{\pi }{3600}rad/s\]
done
clear
D)
\[\frac{\pi }{1800}rad/s\]
done
clear
View Answer play_arrow
question_answer 73) A body is rolling without slipping on a horizontal surface and its rotational kinetic energy is equal to the translational kinetic energy. The body is
A)
disc
done
clear
B)
sphere
done
clear
C)
cylinder
done
clear
D)
ring
done
clear
View Answer play_arrow
question_answer 74) An inelastic ball is dropped from a height of 100 m. Due to collision with earth, 20% of its energy is lost. To what height the ball will rise?
A)
80 m
done
clear
B)
40 m
done
clear
C)
60 m
done
clear
D)
20 m
done
clear
View Answer play_arrow
question_answer 75) If two soap bubbles of equal radii R coalesce then the radius of curvature of interface between two bubbles will be
A)
infinity
done
clear
B)
R
done
clear
C)
R/2
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 76) In the two vessels of same volume, atomic hydrogen and helium at pressures 1 atm and 2 atm are filled. If temperature of both the samples is same, then average speed of hydrogen atoms \[<{{c}_{H}}>\]will be related to that of helium\[<{{c}_{He}}>\]as
A)
\[<{{c}_{H}}>=\sqrt{2}~<{{c}_{He}}>\]
done
clear
B)
\[<{{c}_{H}}>=<{{c}_{He}}>\]
done
clear
C)
\[<{{c}_{H}}>=2<{{c}_{He}}>\]
done
clear
D)
\[<{{c}_{H}}>=\frac{<{{c}_{He}}>}{2}\]
done
clear
View Answer play_arrow
question_answer 77) Presence of the atmosphere at any planet means
A)
\[{{V}_{rms}}<<{{v}_{e}}\]
done
clear
B)
\[{{V}_{rms}}>{{v}_{e}}\]
done
clear
C)
the mass and density of planet is less
done
clear
D)
\[{{V}_{rms}}=0\]
done
clear
View Answer play_arrow
question_answer 78) An astronaut orbiting the earth in a circular orbit 120 km above the surface of earth , gently drops a pen out of space ship. The pen will
A)
move towards the moon
done
clear
B)
fall vertically down to the earth
done
clear
C)
move along with space-ship
done
clear
D)
move opposite direction of space-ship
done
clear
View Answer play_arrow
question_answer 79) An amplitude of motion represent by displacement equation \[y=\frac{1}{\sqrt{a}}\sin \,\omega t\pm \frac{1}{\sqrt{b}}\,\cos \,\omega t,\]
A)
\[\frac{a+b}{ab}\]
done
clear
B)
\[\frac{\sqrt{a}+\sqrt{b}}{ab}\]
done
clear
C)
\[\frac{\sqrt{a}\pm \sqrt{b}}{ab}\]
done
clear
D)
\[\sqrt{\frac{a+b}{ab}}\]
done
clear
View Answer play_arrow
question_answer 80) In sky, a liquid is heated in weightlessness, the heat is transmitted through
A)
conduction
done
clear
B)
convection
done
clear
C)
radiation
done
clear
D)
Neither, because the liquid cannot be heated in weightlessness
done
clear
View Answer play_arrow
question_answer 81) In Young's double slit experiment the type of diffraction is
A)
Fresnel
done
clear
B)
Fraunhoffer
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) Positive rays are beam of
A)
Positrons
done
clear
B)
Protons
done
clear
C)
\[\alpha -\]particles
done
clear
D)
Positive-ions
done
clear
View Answer play_arrow
question_answer 83) Which one is the minimum inresonancing position of series L-C-R circuit?
A)
Current
done
clear
B)
Impedence
done
clear
C)
Reactance
done
clear
D)
Power factor
done
clear
View Answer play_arrow
question_answer 84) Which of the following curves correctly represents the variation of capacitive reactance\[{{X}_{C}}\]with frequency of
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85)
The curve\[{{v}_{m}}T\] for a ideal black body is
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 86) If light to be polarized by reflection then the angle between reflected and refracted rays is
A)
\[\pi \]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[2\pi \]
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 87) During mean life of a radioactive element, the fraction that disintegrates is
A)
\[e\]
done
clear
B)
\[\frac{1}{e}\]
done
clear
C)
\[\frac{e-1}{e}\]
done
clear
D)
\[\frac{e}{e-1}\]
done
clear
View Answer play_arrow
question_answer 88) The order of wavelength of X-rays are
A)
\[{{10}^{-10}}m\]
done
clear
B)
\[{{10}^{-10}}cm\]
done
clear
C)
\[{{10}^{10}}m\]
done
clear
D)
\[{{10}^{10}}cm\]
done
clear
View Answer play_arrow
question_answer 89) The alternating currents respectively are \[{{I}_{1}}={{I}_{0}}\] and and\[{{I}_{2}}={{I}_{0}}\]\[\cos (\omega +\phi ).\]of its root mean square is
A)
1 : 1
done
clear
B)
1 : \[\phi \]
done
clear
C)
1 : 2
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 90) What will be the percentage of radioactive material after 5 half- life?
A)
31%
done
clear
B)
3.125%
done
clear
C)
0.3%
done
clear
D)
1%
done
clear
View Answer play_arrow
question_answer 91) A star of velocity \[{{10}^{6}}m/s\] is going away from earth. If the wavelength of its spectrum line is 5700 \[\overset{o}{\mathop{\text{A}}}\,\], Dopper displacement will be
A)
200\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
19\[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
20 \[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
0.2 \[\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 92) If x = \[\alpha \]sin \[\left( \omega t+\frac{\pi }{6} \right)\]and\[x'=\alpha \,cos\omega t,\]then the resultant phase difference between both will be
A)
\[\pi /3\]
done
clear
B)
\[\pi /6\]
done
clear
C)
\[\pi /2\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 93) A rocket is moving towards the moon with velocity v. Rocket captain sends the electro- magnetic signal of frequency v towards the moon. After reflection from moon, captain receive the signal, the change of apparent frequency will be
A)
\[\frac{cv}{c-v}\]
done
clear
B)
\[\frac{cv}{c-2v}\]
done
clear
C)
\[\frac{2vv}{c}\]
done
clear
D)
\[\frac{2cv}{v}\]
done
clear
View Answer play_arrow
question_answer 94) The displacement of interference waves respectively are\[{{y}_{1}}=4sin\]\[\omega t\]and \[{{y}_{2}}=3\,\sin \left( \omega t+\frac{\pi }{2} \right).\] The amplitude of resultant wave will be
A)
5
done
clear
B)
7
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 95) An iron rod of length L and magnetic moment M is bent in form of a semicircle. Now its magnetic moment will be
A)
\[\frac{2M}{\pi }\]
done
clear
B)
\[\frac{M}{\pi }\]
done
clear
C)
\[M\]
done
clear
D)
\[M\pi \]
done
clear
View Answer play_arrow
question_answer 96) A light wave of speed v is incidence on plane surface, after reflection its speed will be
A)
v
done
clear
B)
\[v/2\]
done
clear
C)
\[v/3\]
done
clear
D)
\[v/4\]
done
clear
View Answer play_arrow
question_answer 97) General isotope in treatment of cancer is
A)
\[{{K}^{40}}\]
done
clear
B)
\[C{{o}^{60}}\]
done
clear
C)
\[S{{r}^{70}}\]
done
clear
D)
\[{{I}^{131}}\]
done
clear
View Answer play_arrow
question_answer 98) 1 volt is equal to
A)
1 eV
done
clear
B)
\[\frac{Joule}{Coulomb}\]
done
clear
C)
\[Joule\,\times \,Coulomb\]
done
clear
D)
\[\frac{Erg}{Coulomb}\]
done
clear
View Answer play_arrow
question_answer 99) If the distance between a point light source and screen is decrease one-third, then the relation between intensity of light \[\left( I \right)\] and initial light\[({{I}_{0}})\] will be
A)
\[I=\frac{{{I}_{0}}}{3}\]
done
clear
B)
\[I=\frac{{{I}_{0}}}{9}\]
done
clear
C)
\[I={{I}_{0}}\]
done
clear
D)
\[I=9{{I}_{0}}\]
done
clear
View Answer play_arrow
question_answer 100) Two siren situated one kilometer apart are producing sound of frequency 330 Hz. An observer starts moving from one siren to the other with a speed of 2 m/s. If the speed of sound be 330 m/s, what will be the beat frequency heard by the observer?
A)
8
done
clear
B)
4
done
clear
C)
6
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 101) The central atom of which molecule has different hybridization from the following similar structure molecules?
A)
\[C{{l}_{2}}O\]
done
clear
B)
\[O{{F}_{2}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 102) The solubility product of calcium sulphate is\[1\times {{10}^{-10}}\]. What will be the concentration of calcium ions in its saturated solution?
A)
\[1\times {{10}^{5}}\]
done
clear
B)
\[1\times {{10}^{-10}}\]
done
clear
C)
\[1\times {{10}^{-5}}\]
done
clear
D)
\[1\times {{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 103) Hydrogen halide reacts with unsymmetrical alkene to form
A)
a primary alkyl halide
done
clear
B)
a gem dihalide
done
clear
C)
a primary or secondary alkyl halide
done
clear
D)
a secondary or tertiary alkyl halide
done
clear
View Answer play_arrow
question_answer 104) Which of the following is not matching? [a] Methane nitrile HCN [b] Iso-butyric acid \[{{(C{{H}_{3}})}_{3}}C-COOH\] [c] Acetonitrile\[C{{H}_{3}}-C-N\] [d] Crotonic acid \[C{{H}_{3}}-CH=CH-COOH\]
A)
A and B
done
clear
B)
A and C
done
clear
C)
A and D
done
clear
D)
B and C
done
clear
View Answer play_arrow
question_answer 105) Which of the following is favourable condition for the formation of ammonia in equilibrium reaction \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}+22\,kcal?\]
A)
increase in pressure
done
clear
B)
increase in temperature
done
clear
C)
decrease in pressure
done
clear
D)
increase in the concentration of ammonia
done
clear
View Answer play_arrow
question_answer 106) For the complete reduction of a molecule of \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]the number of electrons are required
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 107) \[23.0g\]ethanol vapours are passed on hot alumina. If 50% change is completed then the quantity of organic product (molecular weight = 28) in gram will be
A)
14 g
done
clear
B)
7.0 g
done
clear
C)
11 g
done
clear
D)
11.5 g
done
clear
View Answer play_arrow
question_answer 108) Which of the following pair has the same oxidation number of sulphur and chromium?
A)
\[SO_{3}^{2-},CrO_{4}^{2-}\]
done
clear
B)
\[S{{O}_{3}},CrO_{4}^{2-}\]
done
clear
C)
\[S{{O}_{2}},CrO_{4}^{2-}\]
done
clear
D)
\[S{{O}_{2}},C{{r}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 109) Which of the following sodium acetate solution will show minimum pH?
A)
0.01 M
done
clear
B)
0.001 M
done
clear
C)
0.0001 M
done
clear
D)
0.1 M
done
clear
View Answer play_arrow
question_answer 110) Which of the following is not matching?
A)
Isoprene \[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
done
clear
B)
Diacetoneamine
done
clear
C)
Pyrene \[CC{{l}_{4}}\]
done
clear
D)
Chloretone \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-CC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 111) Which is suitable for the test of primary amine?
A)
Chlorine
done
clear
B)
Acetyl chloride
done
clear
C)
Chloroform and alkali
done
clear
D)
Alkyl chloride
done
clear
View Answer play_arrow
question_answer 112) The IUPAC name of \[HO{{H}_{2}}C-C{{H}_{2}}-CH-{{(C{{H}_{2}}OH)}_{2}}\]
A)
3-hydroxy methyl-1, 4-butane diol
done
clear
B)
2-hydroxy methyl-1, 4-butane diol
done
clear
C)
2-hydroxy methyl-4-hydroxy butanol
done
clear
D)
3-hydroxy methyl-4-hydroxy butanol
done
clear
View Answer play_arrow
question_answer 113) Which reagent is used for distinguishing in methanol and ethanol?
A)
Schiff reagent
done
clear
B)
Lucas reagent
done
clear
C)
\[{{I}_{2}}+NaOH\]
done
clear
D)
Chromic acid
done
clear
View Answer play_arrow
question_answer 114) Ethene and ethyne can be distinguished by the reaction of
A)
dil. alkaline \[KMn{{O}_{4}}\]
done
clear
B)
\[{{I}_{2}}\]and\[NaOH\]
done
clear
C)
ammoniacal \[C{{u}_{2}}C{{l}_{2}}\]
done
clear
D)
anhydrous\[ZnC{{l}_{2}}+\]cone.\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 115) The structure of\[PC{{l}_{5}}\]and number of o-bonds in it are
A)
square planar, 5
done
clear
B)
distorted tetrahedral, 4
done
clear
C)
trigonal bipyramidal, 5
done
clear
D)
trigonal pyramidal, 4
done
clear
View Answer play_arrow
question_answer 116) Which compound has octane number 100?
A)
2, 2, 4-trimethyl pentane
done
clear
B)
iso-heptane
done
clear
C)
n-heptane
done
clear
D)
n-octane
done
clear
View Answer play_arrow
question_answer 117) Which of the following gives haloform reaction?
A)
Acetaldehyde
done
clear
B)
Propionaldehyde
done
clear
C)
iso-butyraldehyde
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 118) A carbonyl compound [A] forms compound [B] on reaction with HCN. By the hydrolysis of compound [B], compound [C] is obtained. The compound [C] shows optical isomerism and give iodoform test. Compound [A], is
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
acetone
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) The percentage of nitrogen will be equal in oximes of which of the following compound pairs?
A)
Acetone and acetaldehyde
done
clear
B)
Acetone and propionaldehyde
done
clear
C)
Acetone and acraldehyde
done
clear
D)
Acetaldehyde and acraldehyde
done
clear
View Answer play_arrow
question_answer 120) Benzene reacts with chlorine in the presence of UV-light to give
A)
chlorobenzene
done
clear
B)
hexachlorocyclohexane
done
clear
C)
hexachlorobenzene
done
clear
D)
1, 3, 5-trichlorobenzene
done
clear
View Answer play_arrow
question_answer 121) Which of the following is not matching?
A)
Fool's gold \[Fe{{S}_{2}}\]
done
clear
B)
Philosopher's wool \[ZnO\]
done
clear
C)
Calomel \[H{{g}_{2}}C{{l}_{2}}\]
done
clear
D)
Lunar caustic \[AgCl\]
done
clear
View Answer play_arrow
question_answer 122)
Match the list-I with list-II and choose the correct set from the sets, which are given below List-I List-II [A] Number of sub-energy levels in a energy level 1. \[{{n}^{2}}\] [B] Number of orbitals in a sub-energy level 2. \[3d\] [C] Number of orbitals in a energy level 3. \[2l+1\] [D] \[n=3,l=2,\text{ }m=0\] 4. \[n\]
A)
Codes A-4 B-3 C-1 D-2
done
clear
B)
A-3 B-1 C-2 D-4
done
clear
C)
A- 1 B- 2 C-3 D-4
done
clear
D)
A- 3 B-4 C-1 D-2
done
clear
View Answer play_arrow
question_answer 123) Which of the following compound is colourless and odourless?
A)
\[C{{H}_{3}}NC\]
done
clear
B)
\[CH{{I}_{3}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{F}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 124) Electron affinity is the characteristic of an
A)
isolated atom
done
clear
B)
combined atom
done
clear
C)
gaseous isolated atom in ground state
done
clear
D)
gaseous isolated atom in excited state
done
clear
View Answer play_arrow
question_answer 125) The highest atomic number radioactive element in p-block element is
A)
\[Pb\]
done
clear
B)
\[Te\]
done
clear
C)
\[Rn\]
done
clear
D)
\[Po\]
done
clear
View Answer play_arrow
question_answer 126)
From the following ores, the set of sulphide and carbonate ores is I. Cinnabar II. Calamine III. Copper glance IV. Siderite V. Corundum VI. Magnatite
A)
I, III, V, VI
done
clear
B)
I, II, III, IV
done
clear
C)
I, II, V, VI
done
clear
D)
II, III, IV, V
done
clear
View Answer play_arrow
question_answer 127) Which of the following statement is false?
A)
All methyl ketones give iodoform test
done
clear
B)
All secondary alcohols give iodoform test
done
clear
C)
Methanol forms from the catalytic oxidation of methane
done
clear
D)
Rate of esterification of alcohol \[1{}^\circ >2{}^\circ >3{}^\circ \]
done
clear
View Answer play_arrow
question_answer 128) Which of the following is correctly matched?
A)
Maximum electronegativity element\[-Cl\]
done
clear
B)
Number of d-electrons in chromium\[-6\]
done
clear
C)
Cation with pseudo inert gas structure\[-Z{{n}^{2+}}\]
done
clear
D)
Number of unpaired electrons in\[F{{e}^{3+}}\] ion-4
done
clear
View Answer play_arrow
question_answer 129) Acetaldehyde changes to a sweet-smell liquid on reaction with aluminium ethoxide. This liquid is
A)
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}}O)}_{3}}Al\]
done
clear
View Answer play_arrow
question_answer 130) Which of the following is obtained from the reaction of formaldehyde with dil. alkali?
A)
A polymer
done
clear
B)
Salt of formic acid
done
clear
C)
Methyl alcohol
done
clear
D)
Methyl alcohol and salt of formic acid
done
clear
View Answer play_arrow
question_answer 131) Which of the following reagent releases nitrogen, on reaction with ethyl amine?
A)
Nitrosyi chloride
done
clear
B)
Acetyl chloride
done
clear
C)
Carbon disulphide
done
clear
D)
Benzoyl chloride
done
clear
View Answer play_arrow
question_answer 132) Which of the following is correctly matched? [a]\[{{C}_{6}}{{H}_{6}}\](Benzene) : All C-atom are\[s{{p}^{2}}\] hybridized [b]\[{{(C{{H}_{3}})}_{4}}C:\]All C-atom are\[s{{p}^{3}}\]hybridized [c]\[HC=C-CH=C{{H}_{2}}\]: Four C-atom are sp-hybridized [d] \[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\]: Three C-atom are\[s{{p}^{2}}\]hybridized
A)
A, B
done
clear
B)
A, C
done
clear
C)
A, D
done
clear
D)
B, C
done
clear
View Answer play_arrow
question_answer 133) The equilibrium of aqueous solution of\[{{H}_{2}}S\]is as \[{{H}_{2}}S{{H}^{+}}+H{{S}^{-}}\] On adding\[HCl\]at equilibrium
A)
concentration of\[H{{S}^{-}}\]will increase
done
clear
B)
concentration of\[H{{S}^{-}}\]will decrease
done
clear
C)
dissociation of\[{{H}_{2}}S\]will increase
done
clear
D)
dissociation of\[{{H}_{2}}S\]will stop
done
clear
View Answer play_arrow
question_answer 134) The density of ice is less than water because
A)
ice floats on water
done
clear
B)
the structure of ice is three dimensional with porous
done
clear
C)
H-bond does not present in ice
done
clear
D)
water is a polar solvent
done
clear
View Answer play_arrow
question_answer 135) Which of the following is not correctly matched?
A)
\[CHC{{l}_{3}}+C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow[{}]{{}}\]a solvent
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH+CaOC{{l}_{2}}\xrightarrow[{}]{{}}\]a anaesthetic
done
clear
C)
\[C{{H}_{3}}COH+{{I}_{2}}+NaOH\xrightarrow[{}]{{}}\]a yellow colour precipitate
done
clear
D)
\[CHC{{l}_{3}}+HN{{O}_{3}}\xrightarrow[{}]{{}}\]a war gas
done
clear
View Answer play_arrow
question_answer 136) Which of the following set is correctly matched?
A)
\[n=3,\] \[l=3,m=0,\] \[s=+\frac{1}{2}\]
done
clear
B)
\[n=3,\] \[l=2,m=-3,\] \[s=+\frac{1}{2}\]
done
clear
C)
\[n=3,\] \[l=3,m=-3,\] \[s=-\frac{1}{2}\]
done
clear
D)
\[n=3,\] \[l=2,m=-1,\] \[s=-\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 137) Which of the following is deposited as thin layer in galvanised iron?
A)
Aluminium
done
clear
B)
Zinc
done
clear
C)
Tin
done
clear
D)
White lead
done
clear
View Answer play_arrow
question_answer 138) The value of energy will increase in sub-energy level
A)
on increasing the value of principal quantum number
done
clear
B)
on increasing the value of azimuthal quantum number
done
clear
C)
on increasing the value of both principal quantum number and azimuthal quantum number
done
clear
D)
on increasing the value of spin quantum Number
done
clear
View Answer play_arrow
question_answer 139) The outermost structure of an atom A is\[n{{s}^{2}},n{{p}^{3}}\]. This compound does not form\[A{{F}_{5}}.\] Which of the following statement is false in the given reference?
A)
A is element of second period
done
clear
B)
d-electron is not in A
done
clear
C)
The value of n is less than three
done
clear
D)
A cannot form tetrahedral compound
done
clear
View Answer play_arrow
question_answer 140) Which forms on the reaction of propyne with dil.\[{{H}_{2}}S{{O}_{4}}\] in the presence of mercuric ion?
A)
2-propanol
done
clear
B)
Propanone
done
clear
C)
Propanal
done
clear
D)
1, 2-propane diol
done
clear
View Answer play_arrow
question_answer 141) Which alkene forms only ethanal on ozonolysis?
A)
1-butene
done
clear
B)
2-butene
done
clear
C)
Propene
done
clear
D)
iso-butylene
done
clear
View Answer play_arrow
question_answer 142) Which of the following is correct order of radius?
A)
\[{{K}^{+}}>C{{a}^{2+}}>S{{c}^{3+}}<T{{i}^{4+}}\]
done
clear
B)
\[{{S}^{2-}}>C{{l}^{-}}>Ar<{{K}^{+}}\]
done
clear
C)
\[{{O}^{2-}}>{{F}^{-}}>Ne>N{{a}^{+}}>M{{g}^{2+}}\]
done
clear
D)
\[N{{a}^{+}}>M{{g}^{2+}}>Ne>A{{l}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 143)
A)
phenoxide ion and dichlorocarbene
done
clear
B)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\] and \[{}_{\bullet }^{\bullet }C{{l}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\] and \[{}_{\bullet }^{\bullet }CC{{l}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\] and \[{}_{\bullet }^{\bullet }CHCl\]
done
clear
View Answer play_arrow
question_answer 144) The colour of methyl orange in basic medium is
A)
red
done
clear
B)
colourless
done
clear
C)
yellow
done
clear
D)
orange
done
clear
View Answer play_arrow
question_answer 145) The stability order of carbonium ion is
A)
\[3{}^\circ >2{}^\circ >1{}^\circ >C{{H}_{3}}\]
done
clear
B)
\[3{}^\circ <2{}^\circ >1{}^\circ <C{{H}_{3}}\]
done
clear
C)
\[3{}^\circ <2{}^\circ >1{}^\circ >C{{H}_{3}}\]
done
clear
D)
\[3{}^\circ <2{}^\circ <1{}^\circ <C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 146) The density of Na is higher than K because
A)
ionisation potential of Na is greater than K
done
clear
B)
size of Na is smaller than K
done
clear
C)
atomic weight of K is greater than Na
done
clear
D)
only eight electrons are present in third shell of K
done
clear
View Answer play_arrow
question_answer 147) \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\xrightarrow[{}]{Zn/N{{H}_{4}}Cl}A\] Compound A, is
A)
phenyl hydroxyl amine
done
clear
B)
nitrosobenzene
done
clear
C)
azobenzene
done
clear
D)
p-aminophenol
done
clear
View Answer play_arrow
question_answer 148) \[Ar-CON{{H}_{2}}\xrightarrow[{}]{B{{r}_{2}}+KOH}Ar-N{{H}_{2}}\] This reaction is
A)
Hofmann mustard oil reaction
done
clear
B)
Hofmann bromamide reaction
done
clear
C)
Hofmann martius rearrangement
done
clear
D)
Hofmann carbylamme reaction
done
clear
View Answer play_arrow
question_answer 149) \[C{{H}_{3}}MgX+A\xrightarrow[{}]{{}}B\xrightarrow[{}]{{{H}_{3}}{{O}^{\oplus }}}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\] Compound A, in the above reaction is
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{(C{{H}_{2}})}_{2}}O\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CO\]
done
clear
View Answer play_arrow
question_answer 150) The same magnetic moment ions are
A)
\[C{{u}^{+}},F{{e}^{3+}}\]
done
clear
B)
\[M{{n}^{2+}},F{{e}^{3+}}\]
done
clear
C)
\[C{{u}^{2+}},F{{e}^{2+}}\]
done
clear
D)
\[M{{n}^{2+}},T{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 151) Which of the following presents in sapphine?
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[A{{g}_{2}}O\]
done
clear
C)
\[C{{u}_{2}}O\]
done
clear
D)
\[MnO\]
done
clear
View Answer play_arrow
question_answer 152) The set of oxidation numbers of nitrogen in ammonium nitrate is
A)
\[-3,+3\]
done
clear
B)
\[-1,+1\]
done
clear
C)
\[+1,-1\]
done
clear
D)
\[-3,+5\]
done
clear
View Answer play_arrow
question_answer 153) The formula of microcosmic salt is
A)
\[N{{a}_{2}}HP{{O}_{4}}.2{{H}_{2}}O\]
done
clear
B)
\[NaN{{H}_{4}}HP{{O}_{4}}.4{{H}_{2}}O\]
done
clear
C)
\[{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}N{{H}_{4}}P{{O}_{4}}.2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 154) Which of the following set is correct on the basis of following lists? List-I List-II [A] \[N{{H}_{2}}CONHN{{O}_{2}}\] 1. Biuret [B] \[{{({{C}_{6}}{{H}_{5}}NH)}_{2}}CO\] 2. Semicarbazide [C] \[N{{H}_{2}}CONHN{{H}_{2}}\] 3. Nitrourea [D] \[N{{H}_{2}}CONHCON{{H}_{2}}\] 4. Diphenyl urea
A)
Codes A-3 B-4 C-2 D-1
done
clear
B)
A-3 B-1 C-2 D-4
done
clear
C)
A- 3 B- 2 C-1 D-4
done
clear
D)
A-3 B- 1 C-4 D-2
done
clear
View Answer play_arrow
question_answer 155) For equilibrium reaction,\[{{N}_{2}}+{{O}_{2}}2NO\]at definite temperature\[{{K}_{c}}=121\]. What will be the value of \[{{K}_{c}}\]for following equilibrium reaction \[NO\frac{1}{2}{{N}_{2}}+\frac{1}{2}{{O}_{2}}?\]
A)
\[11\]
done
clear
B)
\[\frac{1}{121}\]
done
clear
C)
\[\frac{1}{11}\]
done
clear
D)
\[121\]
done
clear
View Answer play_arrow
question_answer 156) Molten\[NaCl\]conducts the electricity because it contains
A)
free atom
done
clear
B)
free molecule
done
clear
C)
free ion
done
clear
D)
free electron
done
clear
View Answer play_arrow
question_answer 157) The vapour density of\[PC{{l}_{5}}\]at\[300{}^\circ C\]is 60. Its dissociation percentage will be at this temperature
A)
73
done
clear
B)
77
done
clear
C)
83
done
clear
D)
87
done
clear
View Answer play_arrow
question_answer 158) Starch\[\xrightarrow[{}]{A}\]Maltose\[\xrightarrow[{}]{B}\] Glucose\[\xrightarrow[{}]{C}\] Ethyl alcohol In this reaction A, B, C are respectively
A)
Invertase, zymase, maltase
done
clear
B)
Diastase, maltase, zymase
done
clear
C)
Invertase, maltase, zymase
done
clear
D)
Zymase, maltase, invertase
done
clear
View Answer play_arrow
question_answer 159) The pair of isoelectronic species is
A)
\[{{N}_{2}},CO\]
done
clear
B)
\[CO_{3}^{2-},NO_{3}^{-}\]
done
clear
C)
\[NO_{3}^{-},HCO_{3}^{-}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 160) Which alcohol gives blue colour in Victor Meyer's test?
A)
Butyl alcohol
done
clear
B)
iso-butyl alcohol
done
clear
C)
Secondary butyl alcohol
done
clear
D)
Tertiary butyl alcohol
done
clear
View Answer play_arrow
question_answer 161) Which of the following functional groups set is deactivating and\[m-\]directing?
A)
\[-CN,~-N{{H}_{2}},\,-OH\]
done
clear
B)
\[-CHO,\,-OC{{H}_{3}},-S{{O}_{3}}H\]
done
clear
C)
\[-N{{O}_{2}},\,-CHO,\,-S{{O}_{3}}H\]
done
clear
D)
\[-Cl,~-C{{H}_{3}},\,-NHCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 162) The melting point of\[SnC{{l}_{4}}\]is less than\[SnC{{l}_{2}}\] because in comparison of \[S{{n}^{2+}}\]
A)
the size of\[S{{n}^{4+}}\]is smaller
done
clear
B)
more positive charge on \[S{{n}^{4+}}\]
done
clear
C)
the ionization potential of\[S{{n}^{4+}}\]is more
done
clear
D)
the structure of\[SnC{{l}_{4}}\]is tetrahedral
done
clear
View Answer play_arrow
question_answer 163) Phenol\[\xrightarrow[{}]{NaN{{O}_{2}}+conc.{{H}_{2}}S{{O}_{4}}}\]dark colour\[\xrightarrow[{}]{alkali}\]blue colour This reaction is called
A)
Lederer Mannasse reaction
done
clear
B)
Libermann nitroso reaction
done
clear
C)
Coupling reaction
done
clear
D)
Lucas test
done
clear
View Answer play_arrow
question_answer 164) The atomic number of an element is 20. In which period of Periodic Table it will be placed?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 165) For the dissociation of formic acid, acetic acid and carbonic acid the value of\[p{{K}_{a}}\]is 3.62, 4.74 and 6.3 respectively. Which of the following statement is correct?
A)
Formic acid is the strongest acid
done
clear
B)
Acetic acid is the weakest acid
done
clear
C)
Carbonic acid is the strongest acid
done
clear
D)
Acetic acid is weaker than carbonic acid
done
clear
View Answer play_arrow
question_answer 166) Which of the following acid does not contain \[-COOH\] group?
A)
Lactic acid
done
clear
B)
Barbituric acid
done
clear
C)
Succinic acid
done
clear
D)
Carbonic acid
done
clear
View Answer play_arrow
question_answer 167) Which of the following statement is false?
A)
Electron absorbs energy on transition from lower energy level to higher energy level.
done
clear
B)
Electron cannot fall in nucleus from third energy level.
done
clear
C)
Electron emits energy on transition from higher energy level to lower energy level.
done
clear
D)
Electron emits energy spontaneously in its ground state.
done
clear
View Answer play_arrow
question_answer 168) For the hydrolysis reaction \[C{{N}^{-}}+{{H}_{2}}OHCN+O{{H}^{-}}\] Which of the following relationship is not applicable?
A)
\[{{K}_{h}}=\frac{{{K}_{w}}}{\sqrt{{{K}_{a}}(HCN)}}\]
done
clear
B)
\[h=\sqrt{\frac{{{K}_{h}}}{C}}\]
done
clear
C)
\[pH=\frac{1}{2}p{{K}_{a}}(HCN)\]
done
clear
D)
\[{{H}^{+}}=\sqrt{\frac{{{K}_{w}}\times {{K}_{a}}}{C}}\]
done
clear
View Answer play_arrow
question_answer 169)
Match the given molecule in list-I with given structure in list-II and choose the correct set from the given sets. List-I List-II [A] \[A{{X}_{3}}{{E}_{2}}\] 1. Trigonal pyramidal [B] \[A{{X}_{5}}\] 2. T-structure [C] \[A{{X}_{2}}{{E}_{2}}\] 3. Trigonal bipyramidal [D] \[A{{X}_{3}}E\] 4. Angular
(A = Central atom and E = number of lone pairs electrons) Codes
A)
A-2 B- 3 C-4 D- 1
done
clear
B)
A-1 B-2 C-3 D-4
done
clear
C)
A-3 B-1 C-2 D-4
done
clear
D)
A-4 B-3 C-1 D-2
done
clear
View Answer play_arrow
question_answer 170) The degree of dissociation of A is a in equilibrium reaction,\[A2B+C\]. If initially one mole of A is taken then the total number of moles at equilibrium will be
A)
\[1-\alpha \]
done
clear
B)
\[1+\alpha \]
done
clear
C)
\[1-2\alpha \]
done
clear
D)
\[1+2\alpha \]
done
clear
View Answer play_arrow
question_answer 171) Which of the following has zero dipole moment?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 172) Carbon tetrachloride is used as
A)
anaesthetic and disinfectant
done
clear
B)
coolant and fire extinguisher
done
clear
C)
industrial solvent and fire extinguisher
done
clear
D)
antibiotic and industrial solvent
done
clear
View Answer play_arrow
question_answer 173) \[A+B\xrightarrow[{}]{\Delta }HCOOH\xrightarrow[{}]{{{H}_{2}}S{{O}_{4}}/\Delta }C+D\] A, B, C and D are respectively
A)
\[CO,{{H}_{2}}O,CO,{{H}_{2}}O\]
done
clear
B)
\[CO,{{H}_{2}}O,C{{O}_{2}},{{H}_{2}}\]
done
clear
C)
\[C{{O}_{2}},{{H}_{2}}O,C{{O}_{2}},{{H}_{2}}\]
done
clear
D)
\[C{{O}_{2}},{{H}_{2}}O,CO,{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 174) In the reaction \[H{{C}_{2}}O_{4}^{-}+PO_{4}^{3-}HPO_{4}^{2-}+{{C}_{2}}O_{4}^{2-}.\]Bronsted base is
A)
\[PO_{4}^{3-},{{C}_{2}}O_{4}^{2-}\]
done
clear
B)
\[PO_{4}^{3-},HPO_{4}^{2-}\]
done
clear
C)
\[H{{C}_{2}}O_{4}^{-},HPO_{4}^{2-}\]
done
clear
D)
\[H{{C}_{2}}O_{4}^{-},{{C}_{2}}O_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 175) Phenol on heating with phthalic anhydride and cone.\[{{H}_{2}}S{{O}_{4}}\]gets converted to
A)
phenolphthalein
done
clear
B)
phenetol
done
clear
C)
pinacol
done
clear
D)
o-hydroxy benzoic acid
done
clear
View Answer play_arrow
question_answer 176) Which of the following compound forms mesitylene on distillation with cone.\[{{H}_{2}}S{{O}_{4}}\]?
A)
Acetaldehyde
done
clear
B)
Acetone
done
clear
C)
Acetophenol
done
clear
D)
Acetyl chloride
done
clear
View Answer play_arrow
question_answer 177) The structure of tetrafluoroborate
A)
tetrahedral
done
clear
B)
square planar
done
clear
C)
octahedral
done
clear
D)
trigonal planar
done
clear
View Answer play_arrow
question_answer 178)
Arrange the following acids in decreasing order of their\[p{{K}_{a}}\]values Formic acid (I) Acetic acid (II) Chloro acetic acid (III) Trichloro acetic acid (IV)
A)
IV, III, II, I
done
clear
B)
II, I, IV, III
done
clear
C)
II, I, III, IV
done
clear
D)
I, II, IV, III
done
clear
View Answer play_arrow
question_answer 179) The degree of dissociation of a weak carbonic acid of 0.1 M solution is 10%. The value of dissociation constant of this acid will
A)
\[{{10}^{-5}}\]
done
clear
B)
\[{{10}^{-4}}\]
done
clear
C)
\[{{10}^{-3}}\]
done
clear
D)
\[{{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 180) The structure of some complex ions is given in the following list. A. \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] : square planar B. \[{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}}\] : linear C. \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] : octahedral D. \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\]: tetrahedral Which of the above is not matched?
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 181) The correct set of number of lattice points present in unit cell of a body centred cubic structure and a face centred cubic structure
A)
2, 4
done
clear
B)
4, 2
done
clear
C)
4, 6
done
clear
D)
2, 6
done
clear
View Answer play_arrow
question_answer 182) The solubility product of two sparingly soluble electrolyte AX and BX are\[1\times {{10}^{-8}}\]and\[1\times {{10}^{-10}}\] respectively. Which of the following statement is correct?
A)
Solubility of AX is 100 times more than BX.
done
clear
B)
Solubility of BX is 100 times more than AX.
done
clear
C)
Solubility of AX is 10 times more than BX.
done
clear
D)
Comparison of solubility cannot done from these data
done
clear
View Answer play_arrow
question_answer 183) Which of following are correct matched? [a] Position isomer of isopropyi alcohol \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\] [b] Functional isomer of 1-butadiene \[C{{H}_{3}}-C\equiv C-C{{H}_{3}}\] [c] Position isomer of ethylidene chloride \[Cl-C{{H}_{2}}-C{{H}_{2}}-Cl\] [d] Metamer of ethyl acetate \[H-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-O-C{{H}_{2}}-C{{H}_{2}}C{{H}_{3}}\]
A)
A, B
done
clear
B)
B, C
done
clear
C)
C, D
done
clear
D)
A, D
done
clear
View Answer play_arrow
question_answer 184) Generally, alkenes and alkynes show the following type of reaction
A)
nucleophilic addition
done
clear
B)
electrophilic addition
done
clear
C)
free radical substitution
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 185) Ethyl acetate reacts with hydrazine to give
A)
\[C{{H}_{3}}COONH-N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}COHN-NHCOC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CONH-N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 186) The hydrolysis product of which compound form sodium bisulphite addition product but does not give silver mirror with Tollen's reagent?
A)
\[Cl-C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHC{{l}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C(C{{l}_{2}})C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 187) Which of the following reaction is different from other reaction?
A)
\[2H{{g}^{2+}}\xrightarrow[{}]{{}}Hg_{2}^{2+}\]
done
clear
B)
\[Mg\xrightarrow[{}]{{}}M{{g}^{2+}}\]
done
clear
C)
\[2{{I}^{-}}\xrightarrow[{}]{{}}{{I}_{2}}\]
done
clear
D)
\[Cu_{2}^{2+}\xrightarrow[{}]{{}}2C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 188) The IUPAC name of \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{CH}}\,-{{C}_{2}}{{H}_{5}}\]is
A)
3-ethyl-4-methyl hexane
done
clear
B)
3-methyl-4-ethyl hexane
done
clear
C)
3-ethyl-l, 2-dimethyl pentane
done
clear
D)
3-ethyl-4, 5-dimethyl pentane
done
clear
View Answer play_arrow
question_answer 189) Which of the following statement is false?
A)
\[\alpha -\]butylene and isobutylene are chain isomers.
done
clear
B)
n-propyi alcohol and isopropyi alcohol are not position isomers.
done
clear
C)
Equal molecular weight alcohol and ether are functional isomers.
done
clear
D)
1-alkyne does not show geometrical isomerism.
done
clear
View Answer play_arrow
question_answer 190) After calcination, metal is obtained in the form of
A)
oxide
done
clear
B)
hydrated oxide
done
clear
C)
sulphide
done
clear
D)
carbonate
done
clear
View Answer play_arrow
question_answer 191) The reaction of ether with cold HI is called
A)
Williamson's synthesis
done
clear
B)
Zeravitinof method
done
clear
C)
Zeisel method
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 192) Which gas is evolved on heating acetamide with aqueous alkali?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[NO\]
done
clear
View Answer play_arrow
question_answer 193) Which of the following is not a polymer?
A)
Teflon
done
clear
B)
Nylon
done
clear
C)
Orion
done
clear
D)
Phorone
done
clear
View Answer play_arrow
question_answer 194) \[PC{{l}_{5}}PC{{l}_{3}}+C{{l}_{2}}\] In this reaction, degree of dissociation of \[PC{{l}_{5}}\]is proportional to
A)
\[\sqrt{p}\]
done
clear
B)
\[\frac{1}{\sqrt{p}}\]
done
clear
C)
\[{{p}^{2}}\]
done
clear
D)
\[p\]
done
clear
View Answer play_arrow
question_answer 195) Vinegar is
A)
10% formic acid
done
clear
B)
8-10% acetic acid
done
clear
C)
6-10% ethyl alcohol
done
clear
D)
glacial acetic acid
done
clear
View Answer play_arrow
question_answer 196) Water gas is a mixture of
A)
1 mole CO + 1 mole water vapour
done
clear
B)
1 mole CO + 2 mole\[{{H}_{2}}\]
done
clear
C)
2 mole CO + 1 mole\[{{H}_{2}}\]
done
clear
D)
1 mole CO + 1 mole\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 197) Aniline reacts with cone.\[{{H}_{2}}S{{O}_{4}}\]at\[150{}^\circ C\]to form
A)
sulphanilic acid
done
clear
B)
sulphonic acid
done
clear
C)
o-amino sulphonic acid
done
clear
D)
aniline sulphate
done
clear
View Answer play_arrow
question_answer 198) The pH value of\[{{10}^{-8}}N\,HCl\]is
A)
less than 7
done
clear
B)
more than 7 but less than 8
done
clear
C)
8
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 199) The formula of wavelength of spectral lines for hydrogen atom is
A)
\[\frac{1}{\lambda }=R\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]
done
clear
B)
\[\frac{1}{\lambda }=R\left( \frac{1}{n_{2}^{2}}-\frac{1}{n_{1}^{2}} \right)\]
done
clear
C)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{1}}}-\frac{1}{{{n}_{2}}} \right)}^{2}}\]
done
clear
D)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{2}}}-\frac{1}{{{n}_{1}}} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 200) The relationship between the solubility and solubility product is
A)
\[{{K}_{sp}}={{x}^{x}}{{y}^{y}}{{x}^{x+y}}\]
done
clear
B)
\[{{K}_{sp}}={{s}^{x+y}}\]
done
clear
C)
\[{{K}_{sp}}={{x}^{x}}{{y}^{y}}{{s}^{x-y}}\]
done
clear
D)
\[{{K}_{sp}}={{x}^{2}}{{y}^{2}}{{s}^{x+y}}\]
done
clear
View Answer play_arrow
question_answer 201) The distance between the lines\[7x+24y+3=0\] and\[7x+24y+28=0\]is
A)
1 unit
done
clear
B)
\[1\frac{6}{25}\]units
done
clear
C)
25 units
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 202) The imaginary part of\[sin\text{ }h(x+iy)\]is
A)
\[cos\text{ }x\text{ }sinh\text{ }y\]
done
clear
B)
\[-cosh\text{ }x\text{ }sin\text{ }y\]
done
clear
C)
\[cosh\text{ }x\text{ }sin\text{ }y\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 203) \[{{\left( \frac{1+\sin \theta +i\cos \theta }{1+\sin \theta -i\cos \theta } \right)}^{n}}\]is equal to
A)
\[\cos n\left( \frac{\pi }{2}-\theta \right)+i\sin n\left( \frac{\pi }{2}-\theta \right)\]
done
clear
B)
\[\cos n\left( \frac{\pi }{2}-\theta \right)-i\sin n\left( \frac{\pi }{2}-\theta \right)\]
done
clear
C)
\[\cos n\theta +i\sin n\theta \]
done
clear
D)
\[\sin n\theta +i\cos n\theta \]
done
clear
View Answer play_arrow
question_answer 204) \[\tanh (x+y)\]is equal to
A)
\[\frac{\tanh x+\tanh y}{1-\tanh x\tan y}\]
done
clear
B)
\[\frac{\tanh x+\tanh y}{1+\tanh x\tan y}\]
done
clear
C)
\[\frac{\tanh x-\tanh y}{1-\tanh x\tan y}\]
done
clear
D)
\[\frac{\tanh x-\tanh y}{1+\tanh x\tan y}\]
done
clear
View Answer play_arrow
question_answer 205) The value of\[sinh\text{ }3x\]is
A)
\[3\text{ }sinh\text{ }x-4sin\,{{h}^{3}}x\]
done
clear
B)
\[3\text{ }sinh\text{ }x+4sin\,{{h}^{3}}x\]
done
clear
C)
\[4sin\,{{h}^{3}}x-3\text{ }sinh\text{ }x\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 206) Line\[x+2y-8=0\]is the perpendicular bisector of line AB and point B is (3, 5), then coordinates of A are
A)
(2, 2)
done
clear
B)
(2, 1)
done
clear
C)
(1, 1)
done
clear
D)
(1, 2)
done
clear
View Answer play_arrow
question_answer 207) The points A (8,5),\[B(-7,-5)\]and\[C(-5,5)\]are the vertices of a parallelogram, then its fourth vertex is
A)
(15, 10)
done
clear
B)
\[\left( \frac{3}{2},5 \right)\]
done
clear
C)
(10, 15)
done
clear
D)
\[(-4,\text{ }5)\]
done
clear
View Answer play_arrow
question_answer 208) \[\frac{{{(\cos \theta +i\sin \theta )}^{5}}}{{{(\sin \theta +i\cos \theta )}^{7}}}\]is equal to
A)
\[\sin 12\theta -i\cos 12\theta \]
done
clear
B)
\[-\sin 12\theta +i\cos 12\theta \]
done
clear
C)
\[-\sin 12\theta -i\cos 12\theta \]
done
clear
D)
\[\sin 12\theta +i\cos 12\theta \]
done
clear
View Answer play_arrow
question_answer 209) If\[\sin \alpha +\sin \beta +\sin \gamma =0\]and \[cos\alpha +cos\beta +cos\gamma =0,\]then \[sin\text{ }2\alpha +sin\text{ }2\beta +sin\text{ }2\gamma \]is equal to
A)
\[sin\text{ }2(\alpha +\beta +\gamma )\]
done
clear
B)
\[2sin(\alpha +\beta +\gamma )\]
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 210) If\[2\cos \alpha =a+\left( \frac{1}{a} \right)\]and\[2\cos \beta =b+\left( \frac{1}{b} \right),\]then \[ab+\left( \frac{1}{ab} \right)\]is equal to
A)
\[2\text{ }cos(\alpha +\beta )\]
done
clear
B)
\[2\text{ }\sin (\alpha +\beta )\]
done
clear
C)
\[2\text{ }cos(\alpha -\beta )\]
done
clear
D)
\[4\cos \alpha \cos \beta \]
done
clear
View Answer play_arrow
question_answer 211) If\[A=\left[ \begin{matrix} 0 & 1 \\ 0 & 0 \\ \end{matrix} \right],a,b\]are arbitrary scalars and\[I\]is an unit matrix of order\[2\times 2,\]then\[{{(aI+bA)}^{2}}\]is equal to
A)
\[{{a}^{2}}I+{{b}^{2}}{{A}^{2}}\]
done
clear
B)
\[{{a}^{2}}I+2abA\]
done
clear
C)
\[{{a}^{2}}I+2abA+{{b}^{2}}\]
done
clear
D)
\[{{a}^{2}}I+{{b}^{2}}A\]
done
clear
View Answer play_arrow
question_answer 212) If\[A=\left[ \begin{matrix} 2 & -1 \\ 4 & 2 \\ \end{matrix} \right],B=\left[ \begin{matrix} 2 & 3 \\ 1 & 2 \\ \end{matrix} \right]\]and\[C=\left[ \begin{matrix} 1 & 0 \\ 0 & 1 \\ \end{matrix} \right],\]then\[A+B-C\]is equal to
A)
\[\left[ \begin{matrix} 3 & 2 \\ 5 & 3 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 5 & 2 \\ 5 & 5 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 5 & 2 \\ 3 & 5 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 3 & 2 \\ 5 & 5 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 213) If\[{{A}^{T}}\]and\[{{B}^{T}}\]are transpose matrices of two square matrices A and B of order\[2\times 2,\]then\[{{(AB)}^{T}}\]will be equal to
A)
\[{{A}^{T}}{{B}^{T}}\]
done
clear
B)
\[{{B}^{T}}{{A}^{T}}\]
done
clear
C)
\[A{{B}^{T}}\]
done
clear
D)
\[{{A}^{T}}B\]
done
clear
View Answer play_arrow
question_answer 214) \[{{\tanh }^{-1}}x\] is equal to
A)
\[2\log \frac{1-x}{1+x}\]
done
clear
B)
\[2\log \frac{1+x}{1-x}\]
done
clear
C)
\[\frac{1}{2}\log \frac{1+x}{1-x}\]
done
clear
D)
\[\frac{1}{2}\log \frac{1-x}{1+x}\]
done
clear
View Answer play_arrow
question_answer 215) \[cos\,{{h}^{-1}}x\] is equal to
A)
\[\log \{x+\sqrt{{{x}^{2}}+1}\}\]
done
clear
B)
\[\log \{x+\sqrt{{{x}^{2}}-1}\}\]
done
clear
C)
\[\frac{1}{2}\log \{x+\sqrt{{{x}^{2}}+1}\}\]
done
clear
D)
\[\frac{1}{2}\log \{x+\sqrt{{{x}^{2}}-1}\}\]
done
clear
View Answer play_arrow
question_answer 216) The real part of\[{{\cos }^{-1}}(\cos \theta +i\sin \theta )\]is
A)
\[{{\cos }^{-1}}\sqrt{(\sin \theta )}\]
done
clear
B)
\[{{\sin }^{-1}}\sqrt{(\sin \theta )}\]
done
clear
C)
\[\sqrt{\theta }\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 217) The equation of line passes through the point (1,2) and perpendicular to the line\[x+y+1=0,\]is
A)
\[y-x+1=0\]
done
clear
B)
\[y-x+2=0\]
done
clear
C)
\[y-x-1=0\]
done
clear
D)
\[y-x-2=0\]
done
clear
View Answer play_arrow
question_answer 218) The value of the determinant \[\left| \begin{matrix} a+1 & 1 & 1 \\ 1 & b+1 & 1 \\ 1 & 1 & c+1 \\ \end{matrix} \right|\]is
A)
\[abc\]
done
clear
B)
\[ab+bc+ca+abc\]
done
clear
C)
\[ab+bc+ca\]
done
clear
D)
\[3(a+b+c)\]
done
clear
View Answer play_arrow
question_answer 219) If\[{{(1+i\sqrt{3})}^{9}}=a+ib,a,b\in R,\]then the value of \[b\]is
A)
29
done
clear
B)
\[-29\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 220) \[{{(1+\cos \theta +i\sin \theta )}^{3}}+{{(1-\cos \theta -i\sin \theta )}^{3}}\]is equal to
A)
\[{{2}^{4}}{{\cos }^{3}}\left( \frac{\theta }{2} \right)\cos \left( \frac{3\theta }{2} \right)\]
done
clear
B)
\[{{2}^{3}}{{\cos }^{3}}\left( \frac{\theta }{2} \right)\cos \left( \frac{3\theta }{2} \right)\]
done
clear
C)
\[{{2}^{3}}{{\cos }^{3}}\left( \frac{\theta }{2} \right)\sin \left( \frac{3\theta }{2} \right)\]
done
clear
D)
\[{{2}^{4}}{{\cos }^{3}}\left( \frac{\theta }{2} \right)\sin \left( \frac{3\theta }{2} \right)\]
done
clear
View Answer play_arrow
question_answer 221) If\[A=\left[ \begin{matrix} 1 & -2 & 3 \\ 0 & 2 & -1 \\ -2 & 5 & 2 \\ \end{matrix} \right],\]then adj A is
A)
\[\left[ \begin{matrix} 9 & 19 & -4 \\ -4 & 8 & -1 \\ 4 & -1 & 2 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 9 & 19 & 4 \\ -2 & 14 & 1 \\ -4 & 1 & x \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 9 & 19 & -4 \\ 2 & 8 & 1 \\ 4 & -1 & 2 \\ \end{matrix} \right]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 222) The value of\[\left| \begin{matrix} a-b & b-c & c-a \\ b-c & c-a & a-b \\ c-a & a-b & b-c \\ \end{matrix} \right|\]is
A)
abc
done
clear
B)
2abc
done
clear
C)
4abc
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 223) How many numbers greater than 40000 can be formed from the digits 2, 4, 5, 5, 7?
A)
12
done
clear
B)
24
done
clear
C)
36
done
clear
D)
48
done
clear
View Answer play_arrow
question_answer 224) If sum of n terms of an AP is\[2{{n}^{2}}+5n,\]then its nth term is
A)
\[4n+3\]
done
clear
B)
\[4n+5\]
done
clear
C)
\[4n+7\]
done
clear
D)
\[4n+6\]
done
clear
View Answer play_arrow
question_answer 225) Harmonic mean of any two numbers is 4 and relation between their geometric mean and arithmetic mean is\[{{G}^{2}}+2A=27,\]then these numbers are
A)
6, 3
done
clear
B)
4, 27
done
clear
C)
6, 7
done
clear
D)
3, 9
done
clear
View Answer play_arrow
question_answer 226) In a complex plane, the point z moves such that\[|z-3i|=2\]then its locus is
A)
a straight line
done
clear
B)
a circle
done
clear
C)
y-axis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 227) The solutions of\[|{{x}^{2}}-x-6|=x+2\]are
A)
\[-2,\text{ }2,\text{ }4\]
done
clear
B)
\[-2,\text{ }2,-4\]
done
clear
C)
4, 3, 3
done
clear
D)
\[3,\text{ }2,-2\]
done
clear
View Answer play_arrow
question_answer 228) The square root of\[5-12i\]is
A)
\[\pm (3+2i)\]
done
clear
B)
\[\pm (3-2i)\]
done
clear
C)
\[\pm (2+3i)\]
done
clear
D)
\[\pm (2-3i)\]
done
clear
View Answer play_arrow
question_answer 229) In a GP, second term is 2 and sum of infinite terms is 8, then its first term will be
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 230) The roots of the equations\[{{x}^{2}}+ax+b=0\]and \[{{x}^{2}}+bx+a=0\]are common and\[a\ne b,\]then\[a+b\]is equal to
A)
0
done
clear
B)
1
done
clear
C)
\[-1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 231) In the expansion of\[{{\left( \frac{2{{x}^{2}}}{3}+\frac{3}{2{{x}^{2}}} \right)}^{10}},\]the middle term is
A)
251
done
clear
B)
252
done
clear
C)
250
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 232) The quadratic equation of real coefficients, whose one root is\[7+5i,\]will be
A)
\[{{x}^{2}}+14x+74=0\]
done
clear
B)
\[{{x}^{2}}-14x-74=0\]
done
clear
C)
\[{{x}^{2}}+14x-74=0\]
done
clear
D)
\[{{x}^{2}}-14x+74=0\]
done
clear
View Answer play_arrow
question_answer 233) If in the expansion of\[{{(x+a)}^{n}},\]the sum of odd terms is P and sum of even terms is Q, then the value of\[{{({{x}^{2}}-{{a}^{2}})}^{n}}\]is
A)
\[{{P}^{2}}+{{Q}^{2}}\]
done
clear
B)
\[4PQ\]
done
clear
C)
\[{{P}^{2}}-{{Q}^{2}}\]
done
clear
D)
\[2P-2Q\]
done
clear
View Answer play_arrow
question_answer 234) The value of determinant\[\left| \begin{matrix} 4 & -6 & 1 \\ -1 & -1 & 1 \\ -4 & 11 & -1 \\ \end{matrix} \right|\]is
A)
25
done
clear
B)
\[-25\]
done
clear
C)
0
done
clear
D)
\[-75\]
done
clear
View Answer play_arrow
question_answer 235) There are 10 points in a plane. If 4 points are collinear, then the number of straight lines obtained by joining these points is
A)
44
done
clear
B)
45
done
clear
C)
46
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 236) The value of\[cos\,{{h}^{2}}x-sin\,{{h}^{2}}x\]is
A)
\[cos\,h\text{ }2x\]
done
clear
B)
1
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 237) If\[{{y}^{2}}+xy-{{x}^{2}}=5,\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{2x+y}{x+2y}\]
done
clear
B)
\[\frac{2y+x}{2x-y}\]
done
clear
C)
\[\frac{2x-y}{2y+x}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 238) If\[f(x)=\left\{ \begin{matrix} x+1 & when\,x<2 \\ 2x-1, & when\,x\ge 2 \\ \end{matrix} \right.,\]then at\[a=2,f'(x)\]is equal to
A)
10
done
clear
B)
1
done
clear
C)
2
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 239) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{|x|}{x}\]is equal to
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 240) The equation of the normal to the curve\[{{y}^{2}}=4\text{ }ax\]at point\[(a{{t}^{2}},2at)\]is
A)
\[y-tx=a{{t}^{3}}+2at\]
done
clear
B)
\[y+tx=a{{t}^{3}}+2at\]
done
clear
C)
\[y-tx=a{{t}^{3}}-2at\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 241) Function\[f(x)=|x|\]is
A)
discontinuous at\[x=0\]
done
clear
B)
discontinuous at \[x=1\]
done
clear
C)
continuous at all points
done
clear
D)
discontinuous at all points
done
clear
View Answer play_arrow
question_answer 242) Function is defined as\[f:x\to \frac{1+x}{1-x},\]then\[f\{f(x)\}\]is equal to
A)
\[x\]
done
clear
B)
\[\frac{1}{x}\]
done
clear
C)
\[-x\]
done
clear
D)
\[-\frac{1}{x}\]
done
clear
View Answer play_arrow
question_answer 243) If\[y={{\sec }^{-1}}x,\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{1}{x\sqrt{{{x}^{2}}+1}}\]
done
clear
B)
\[\frac{1}{\sqrt{{{x}^{2}}-1}}\]
done
clear
C)
\[\frac{1}{x\sqrt{1-{{x}^{2}}}}\]
done
clear
D)
\[\frac{1}{x\sqrt{{{x}^{2}}-1}}\]
done
clear
View Answer play_arrow
question_answer 244) The centroid of the triangle formed by the lines\[x=0,x-y=3\]and\[2x+3y=6\]is
A)
\[(3,-1)\]
done
clear
B)
\[(1,-3)\]
done
clear
C)
\[\left( 1,\frac{1}{3} \right)\]
done
clear
D)
\[\left( 1,-\frac{1}{3} \right)\]
done
clear
View Answer play_arrow
question_answer 245) Three lines\[3x+4y+6=0,\]\[\sqrt{2}x+\sqrt{3}y+2\sqrt{2}=0\]and\[4x+7y+8=0\]are
A)
sides of a triangle
done
clear
B)
concurrent
done
clear
C)
parallel
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 246) The equation of circle concentric to the circle \[{{x}^{2}}+{{y}^{2}}-3x+4y-2=0\]and passes through the point\[(-1,-2)\]is
A)
\[{{x}^{2}}+{{y}^{2}}-3x-4y=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}+3x-4y=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}-3x+4y+\frac{25}{2}=0\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 247) If\[f(x)=\left\{ \begin{matrix} x, & x<0 \\ 1, & x=0 \\ {{x}^{2}}, & x>0 \\ \end{matrix} \right.,\]then the true statement is
A)
\[\underset{x\to 0}{\mathop{\lim }}\,f(x)=1\]
done
clear
B)
\[\underset{x\to 0}{\mathop{\lim }}\,f(x)=0\]
done
clear
C)
at\[x=0,f(x)\]is continuous
done
clear
D)
\[\underset{x\to 0}{\mathop{\lim }}\,f(x)\]does not exist
done
clear
View Answer play_arrow
question_answer 248) \[\underset{x\to a}{\mathop{\lim }}\,\frac{{{x}^{n}}-{{a}^{n}}}{x-a}\]is equal to
A)
0
done
clear
B)
\[n{{a}^{n-1}}\]
done
clear
C)
\[n{{a}^{n}}\]
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 249) The polar of the circle \[{{x}^{2}}+{{y}^{2}}+2\lambda x+2\mu y+c=0\]with respect to origin will touch\[{{x}^{2}}+{{y}^{2}}={{r}^{2}},\]if
A)
\[{{r}^{2}}={{c}^{2}}({{\lambda }^{2}}+{{\mu }^{2}})\]
done
clear
B)
\[{{c}^{2}}={{r}^{2}}({{\lambda }^{2}}+{{\mu }^{2}})\]
done
clear
C)
\[c=r({{\lambda }^{2}}+{{\mu }^{2}})\]
done
clear
D)
\[r=c({{\lambda }^{2}}+{{\mu }^{2}})\]
done
clear
View Answer play_arrow
question_answer 250) Equation of radical axis of the circles \[{{x}^{2}}+{{y}^{2}}+4x+7=0\]and\[2{{x}^{2}}+2{{y}^{2}}+3x+5y+9=0\]is
A)
\[x+y+1=0\]
done
clear
B)
\[x+y-1=0\]
done
clear
C)
\[x-y-1=0\]
done
clear
D)
\[x-y+1=0\]
done
clear
View Answer play_arrow
question_answer 251) Line\[y=mx+\frac{a}{m}\]is a tangent to the parabola \[{{y}^{2}}=4\text{ }ax,\]then the point of contact is
A)
\[\left( \frac{a}{{{m}^{2}}},\frac{-2a}{m} \right)\]
done
clear
B)
\[\left( \frac{a}{{{m}^{2}}},\frac{2a}{m} \right)\]
done
clear
C)
\[(a{{m}^{2}},2am)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 252) The coordinates of two points A and B are \[(-1,2)\]and\[(2,-1)\]respectively, then equation of circle AB as diameter, is
A)
\[{{x}^{2}}+{{y}^{2}}-x+y-4=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}+x+y-4=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}-x-y-4=0\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 253) The equation of line joining the point (2, 3) to the point of intersection\[2x+3y+1=0\]and \[3x-4y=8,\]is
A)
\[3x+y-9=0\]
done
clear
B)
\[5x-y-7=0\]
done
clear
C)
\[5x+y-13=0\]
done
clear
D)
\[3x-y-3=0\]
done
clear
View Answer play_arrow
question_answer 254) The equation of a parabola is\[{{x}^{2}}=-10y,\]then coordinates of focus are
A)
\[\left( 0,-\frac{5}{2} \right)\]
done
clear
B)
\[\left( 0,\frac{5}{2} \right)\]
done
clear
C)
\[\left( \frac{5}{2},0 \right)\]
done
clear
D)
\[\left( -\frac{5}{2},0 \right)\]
done
clear
View Answer play_arrow
question_answer 255) The equation of tangent to the parabola \[{{y}^{2}}=8x\]which is perpendicular to the line \[2x-y+1=0,\]is
A)
\[x+2y=8\]
done
clear
B)
\[x+2y+8=0\]
done
clear
C)
\[2x+y=8\]
done
clear
D)
\[2x+y+8=0\]
done
clear
View Answer play_arrow
question_answer 256) If vertex and focus of a parabola are (3, 3) and \[(-3,3),\]then its equation is
A)
\[{{(y+3)}^{2}}\]\[~=24(x+3)\]
done
clear
B)
\[{{(y+3)}^{2}}=-24(x+3)\]
done
clear
C)
\[{{(y-3)}^{2}}=-24(x-3)\]
done
clear
D)
\[{{(y-3)}^{2}}=24(x-3)\]
done
clear
View Answer play_arrow
question_answer 257) The equation of normal to the circle \[{{x}^{2}}+{{y}^{2}}-4x+6y=0\]at point (0, 0) is
A)
\[3x-2y=0\]
done
clear
B)
\[3x+2y=0\]
done
clear
C)
\[2x-3y=0\]
done
clear
D)
\[2x+3y=0\]
done
clear
View Answer play_arrow
question_answer 258) A circle touches the line\[2x-y-1=0\]at point (3,5). Its centre lies on the line\[x+y=5,\]then the coordinates of centre of circle are
A)
\[(-3,\text{ }8)\]
done
clear
B)
(2, 3)
done
clear
C)
(3, 2)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 259) If ordinate of a point on the parabola\[{{y}^{2}}=64x\]is 8, then the equation of tangent at this point is
A)
\[4x-y+4=0\]
done
clear
B)
\[4x+y+4=0\]
done
clear
C)
\[x+4y-4=0\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 260) The length of the double ordinate of the parabola\[{{x}^{2}}=8y\]is 8. Then, the end points of this double ordinate are
A)
\[(4,2),(4,-2)\]
done
clear
B)
\[(2,4),(2,-4)\]
done
clear
C)
\[(4,2),(-4,2)\]
done
clear
D)
\[(-2,4),(2,4)\]
done
clear
View Answer play_arrow
question_answer 261) The area of the region bounded by the curve\[y=\frac{3}{{{x}^{2}}},\]\[x-\]axis and\[x=1,\text{ }x=2\]is
A)
\[1\,sq\,unit\]
done
clear
B)
\[3\,sq\,unit\]
done
clear
C)
\[2\,sq\,unit\]
done
clear
D)
\[\frac{3}{2}\,sq\,unit\]
done
clear
View Answer play_arrow
question_answer 262) The area of the region bounded by the curve \[y=4+3x-{{x}^{2}}\]and\[x-\]axis is
A)
\[\frac{125}{6}\]sq units
done
clear
B)
\[\frac{125}{3}\]sq units
done
clear
C)
\[\frac{125}{2}\] sq units
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 263) \[\int_{-1}^{1}{x\,{{\tan }^{-1}}x\,dx}\]is equal to
A)
\[-\frac{\pi }{2}-1\]
done
clear
B)
\[\frac{\pi }{2}+1\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 264) The value of\[\int{\frac{dx}{\sqrt{(3-5x-{{x}^{2}})}}}\]is
A)
\[{{\sin }^{-1}}\left( \frac{2x+5}{\sqrt{37}} \right)+c\]
done
clear
B)
\[{{\sinh }^{-1}}\left( \frac{2x+5}{\sqrt{37}} \right)+c\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{2x+5}{37} \right)+c\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 265) \[\int{\frac{{{e}^{x}}}{{{e}^{x}}-1}}dx\]is equal to
A)
\[\log ({{e}^{x}}-1)+c\]
done
clear
B)
\[-\log ({{e}^{x}}-1)+c\]
done
clear
C)
\[\log (1-{{e}^{x}})+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 266) The value of \[\int_{2}^{4}{\frac{\sqrt{{{x}^{2}}-4}}{x}}dx\] is
A)
\[2\sqrt{3}+\frac{2\pi }{3}\]
done
clear
B)
\[2\sqrt{3}-\frac{\pi }{3}\]
done
clear
C)
\[2\sqrt{3}+\frac{\pi }{3}\]
done
clear
D)
\[2\sqrt{3}-\frac{2\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 267) The value of\[\int_{0}^{2/3}{\frac{dx}{4+9{{x}^{2}}}}\]is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\pi \]
done
clear
C)
\[0\]
done
clear
D)
\[\frac{\pi }{24}\]
done
clear
View Answer play_arrow
question_answer 268) \[\int{\frac{t}{{{e}^{3{{t}^{2}}}}}}dt\]is equal to
A)
\[\frac{1}{6}{{e}^{3{{t}^{2}}}}+c\]
done
clear
B)
\[-\frac{1}{5}{{e}^{3{{t}^{2}}}}+c\]
done
clear
C)
\[\frac{1}{6}{{e}^{-3{{t}^{2}}}}+c\]
done
clear
D)
\[-\frac{1}{6}{{e}^{-3{{t}^{2}}}}+c\]
done
clear
View Answer play_arrow
question_answer 269) The area of the region bounded by the curve \[y=cos\text{ }x\]and\[x-\]axis when\[0<x\le 2\pi ,\]will be
A)
4 sq units
done
clear
B)
3 sq units
done
clear
C)
2 sq units
done
clear
D)
1 sq unit
done
clear
View Answer play_arrow
question_answer 270) If\[y=log\text{ }{{x}^{10}},\]then\[\frac{dy}{dx}\]is equal to
A)
\[10{{x}^{2}}\]
done
clear
B)
\[\frac{10}{x}\]
done
clear
C)
\[10x\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 271) \[{{e}^{x}}\sin y-{{e}^{y}}\cos x=1,\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{\sin y+{{e}^{y-x}}\sin x}{\cos y-{{e}^{y-x}}\cos x}\]
done
clear
B)
\[\frac{\cos y-{{e}^{y-x}}\cos x}{\sin y+{{e}^{y-x}}\sin x}\]
done
clear
C)
\[\frac{\cos y+{{e}^{y-x}}\cos x}{\sin y-{{e}^{y-x}}\sin x}\]
done
clear
D)
\[\frac{\sin y+{{e}^{y-x}}\sin x}{{{e}^{y-x}}\cos x-\cos y}\]
done
clear
View Answer play_arrow
question_answer 272) If\[y=\frac{\sec x+\tan x}{\sec x-\tan x},\]then\[\frac{dy}{dx}\]is equal to
A)
\[2\text{ }sec\text{ }x{{(sec\text{ }x+tan\text{ }x)}^{2}}\]
done
clear
B)
\[2\text{ }sec\text{ }x{{(sec\text{ }x-tan\text{ }x)}^{2}}\]
done
clear
C)
\[2\,sec\,x\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 273) The equation of the normal to the curve \[y=x(2-x)\]at point (2, 0) is
A)
\[x-2y=2\]
done
clear
B)
\[x-2y+2=0\]
done
clear
C)
\[2x+y=0\]
done
clear
D)
\[2x+y-4=0\]
done
clear
View Answer play_arrow
question_answer 274) The length of subnormal to the curve\[{{y}^{2}}=4x\] at any point is
A)
2
done
clear
B)
\[\frac{{{y}^{2}}}{2}\]
done
clear
C)
\[2{{y}^{2}}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 275) If\[x=a{{t}^{2}},\text{ }y=2at,\]then the value of\[\frac{dy}{dx}\]at\[t=2\] is
A)
2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 276) If\[y=\sqrt{\left( \frac{1+x}{1-x} \right)},\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{y}{1-{{x}^{2}}}\]
done
clear
B)
\[\frac{y}{{{x}^{2}}-1}\]
done
clear
C)
\[\frac{y}{{{x}^{2}}+1}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 277) If\[y={{(x-2)}^{x}},\]then\[\frac{dy}{dx}\]is equal to
A)
\[{{(x-2)}^{x}}\log (x-2)+x{{(x-2)}^{x-1}}\]
done
clear
B)
\[{{(x-2)}^{x}}\log (x-2)+{{(x-2)}^{x}}\]
done
clear
C)
\[{{(x-2)}^{x-1}}\log (x-1)+x{{(x-2)}^{x-1}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 278) The points on the curve\[y=12x-{{x}^{3}}\]at which the slope of the tangent will be zero, are
A)
\[(2,-16),(-2,16)\]
done
clear
B)
(3, 10), (0, 10)
done
clear
C)
\[(2,16),(-2,-16)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 279) The value of\[\int{{{\sec }^{4}}xdx}\]is
A)
\[\tan x+\frac{1}{3}{{\sec }^{3}}x+c\]
done
clear
B)
\[\frac{1}{6}\tan x+{{\sec }^{3}}x+c\]
done
clear
C)
\[\tan x-\frac{1}{3}{{\sec }^{3}}x+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 280) The function\[f(x)=\frac{1}{3}{{x}^{3}}+\frac{1}{2}{{x}^{2}}-6x+8\]is decreasing in the interval
A)
\[(-\infty ,-3)\]
done
clear
B)
\[(2,\infty )\]
done
clear
C)
\[(-3,2)\]
done
clear
D)
\[(-\infty ,-3)\cup (2,\infty )\]
done
clear
View Answer play_arrow
question_answer 281) The point of inflexion on the curve\[y={{x}^{5/3}}\] will be
A)
(0, 0)
done
clear
B)
(1, 0)
done
clear
C)
(0, 1)
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 282) The value of\[\int{\frac{{{({{x}^{2}}+8)}^{2}}}{{{x}^{4}}}}dx\]is equal to
A)
\[x+\frac{16}{x}+\frac{64}{3{{x}^{2}}}+c\]
done
clear
B)
\[x-\frac{16}{x}-\frac{64}{3{{x}^{3}}}+c\]
done
clear
C)
\[x-\frac{32}{{{x}^{3}}}-\frac{256}{3{{x}^{2}}}+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 283) The value of\[\int{\frac{\tan xdx}{\sec x+\cos x}}\]is
A)
\[{{\tan }^{-1}}(\cos x)+c\]
done
clear
B)
\[-{{\tan }^{-1}}(\cos x)+c\]
done
clear
C)
\[{{\tan }^{-1}}(\sin x)+c\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 284) If\[x\]and y are two variables such that\[xy=1,\text{ }x>1,\]then the minimum value of\[x+y\] will be
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 285) The maximum value of\[sin\text{ }x+cos\text{ }x\]is
A)
\[2\]
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 286) The value of\[\int{{{\sec }^{3}}\theta d\theta }\]is
A)
\[\frac{1}{2}log\text{(}sec\theta +tan\theta )+\frac{1}{2}sec\theta tan\theta +c\]
done
clear
B)
\[log(sec\theta +tan\theta )+sec\theta tan\theta +c\]
done
clear
C)
\[log(sec\theta -tan\theta )+\frac{1}{2}sec\theta tan\theta +c\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 287) The value of\[\int{\frac{4x-7}{{{x}^{2}}+x-2}}dx\]is
A)
\[\log ({{x}^{2}}+x-2)+3\left( \frac{x-1}{x+2} \right)+c\]
done
clear
B)
\[2\log ({{x}^{2}}+x-2)-3\log \left( \frac{x+1}{x+2} \right)+c\]
done
clear
C)
\[2\log ({{x}^{2}}+x-2)-3\log \left( \frac{x-1}{x+2} \right)+c\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 288) A man draws a card from a pack of 52 playing cards, replace it and shuffles the pack. He continues this processes until he gets a card of spade. The probability that he will fail the first two times, is
A)
\[\frac{9}{64}\]
done
clear
B)
\[\frac{1}{64}\]
done
clear
C)
\[\frac{1}{16}\]
done
clear
D)
\[\frac{9}{16}\]
done
clear
View Answer play_arrow
question_answer 289) Two dice are thrown together. The probability of getting the sum 7 is
A)
\[\frac{7}{36}\]
done
clear
B)
\[\frac{1}{36}\]
done
clear
C)
\[\frac{1}{6}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 290) The projection of vector\[2\hat{i}+3\hat{j}-2\hat{k}\] on the vector \[\hat{i}+2\hat{j}+3\hat{k}\]is
A)
\[\frac{1}{\sqrt{14}}\]
done
clear
B)
\[\frac{2}{\sqrt{14}}\]
done
clear
C)
\[\frac{2}{\sqrt{17}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 291) The probability that a leap year will have 53 Sundays, is
A)
\[\frac{2}{7}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{3}{5}\]
done
clear
D)
\[\frac{1}{7}\]
done
clear
View Answer play_arrow
question_answer 292) If\[[\overrightarrow{a}+\overrightarrow{b}]=[\overrightarrow{a}-\overrightarrow{b}],\]then the vectors\[\overrightarrow{a}\]and\[\overrightarrow{b}\]are
A)
parallel
done
clear
B)
perpendicular
done
clear
C)
collinear
done
clear
D)
coplanar
done
clear
View Answer play_arrow
question_answer 293) If the adjacent sides of a parallelogram are represented by\[\overrightarrow{a}=3\hat{i}+\hat{j}+2\hat{k}\]and \[\overrightarrow{b}=2\hat{i}-2\hat{j}+4\hat{k},\]then its area will be
A)
\[8\sqrt{3}\] sq units
done
clear
B)
8 sq units
done
clear
C)
\[8\sqrt{2}\]sq units
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 294) If\[\overrightarrow{a}=\hat{i}+2\hat{j}-\hat{k}\]and\[\overrightarrow{b}=2\hat{i}+\hat{j}+\hat{k},\]then the angle between 1st and b will be
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 295) Six boys and six girls are sitting in a row. The probability that all 6 girls sit together, is
A)
\[\frac{1}{102}\]
done
clear
B)
\[\frac{1}{112}\]
done
clear
C)
\[\frac{1}{122}\]
done
clear
D)
\[\frac{1}{132}\]
done
clear
View Answer play_arrow
question_answer 296) The value of\[\underset{x\to 0}{\mathop{\lim }}\,\left[ \frac{{{e}^{x}}-{{e}^{\sin x}}}{x-\sin x} \right]\]is
A)
\[-1\]
done
clear
B)
0
done
clear
C)
1
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 297) The number of terms in the expansion of \[{{(a+b+c)}^{10}}\]is
A)
11
done
clear
B)
21
done
clear
C)
55
done
clear
D)
66
done
clear
View Answer play_arrow
question_answer 298) The eccentricity of the conjugate hyperbola of the hyperbola\[{{x}^{2}}-3{{y}^{2}}=1\]is
A)
\[\frac{4}{3}\]
done
clear
B)
4
done
clear
C)
\[2\]
done
clear
D)
\[\frac{2}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 299) The value of\[\int_{0}^{\pi }{\frac{x\,dx}{1+\sin x}}\]is
A)
\[-\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 300) Harmonic mean of the two numbers\[\frac{a}{1-ab}\]and \[\frac{a}{1+ab}\]is
A)
\[\frac{1}{1-{{a}^{2}}{{b}^{2}}}\]
done
clear
B)
\[\frac{a}{1-{{a}^{2}}{{b}^{2}}}\]
done
clear
C)
\[a\]
done
clear
D)
\[\frac{a}{\sqrt{1-{{a}^{2}}{{b}^{2}}}}\]
done
clear
View Answer play_arrow
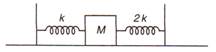
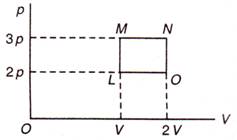
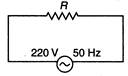
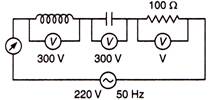
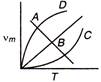
 The intermediates are formed in this reaction are
The intermediates are formed in this reaction are
