A) -5J
B) -10 J
C) -20 J
D) -15 J
Correct Answer: A
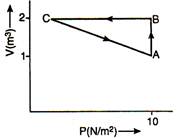
Solution :
From first law of thermodynamics \[\Delta U=Q-W\] For cycle ABCA, \[\Delta U=0\] \[\therefore \] \[Q=W={{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}\] \[\Rightarrow \] \[5=10+0+{{W}_{CA}}\] \[\therefore \] \[{{W}_{CA}}=-5\,\text{J}\]You need to login to perform this action.
You will be redirected in
3 sec
