A) \[4,2\]
B) \[3,2\]
C) \[4,3\]
D) None of these
Correct Answer: A
Solution :
\[SO_{4}^{2-}\] ion has following structure: The number of \[\sigma -\] bonds \[=4\] The number of \[\pi -\]bonds \[=2\]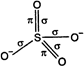
You need to login to perform this action.
You will be redirected in
3 sec
