A) pemene-1
B) propene
C) 2-butene
D) butene-1
Correct Answer: C
Solution :
For a compound to exhibit geometrical isomerism it is necessary that it has (i) Atleast one carbon-carbon double bond. (ii) The two group attached to same carbon atom are different. From the choices find the one which satisfies both the conditions. (a)\[\underset{pentene-1}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}=C{{H}_{2}}}}\,\] (b)\[\underset{propene}{\mathop{C{{H}_{3}}-CH=C{{H}_{2}}}}\,\] (d)\[\underset{1-butene}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}}}\,\] In choices (a), (b) and (d), two groups attached to same carbon atom are different. So they will not show geometrical isomerism.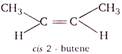
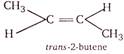 \[\because \,\,2-\]butene satisfies both the conditions. \[\therefore \]It exists in two structural forms and shows geometrical isomerism.
\[\because \,\,2-\]butene satisfies both the conditions. \[\therefore \]It exists in two structural forms and shows geometrical isomerism.
You need to login to perform this action.
You will be redirected in
3 sec
