A) \[CuCl_{4}^{2}-1\]unpaired electron
B) \[[Fe{{({{H}_{2}}{{O}_{6}}]}^{2+}}-5\]unpaired electrons
C) \[{{[Zn{{(N{{H}_{3}})}_{2}}]}^{2+}}\]Diamagnetic
D) \[{{[Co{{F}_{6}}]}^{3-}}-4\]unpaired electrons
Correct Answer: B
Solution :
In \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\] the \[Fe(II)\] ion has \[3{{d}^{6}}\] electronic configuration and pairing of electron in \[3d\] orbital does not take place due to the presence of weak field ligand\[{{H}_{2}}O\].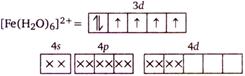 Hence, there are four unpaired electrons in \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]ion.
Hence, there are four unpaired electrons in \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]ion.
You need to login to perform this action.
You will be redirected in
3 sec
