A) strong field
B) weak field
C) neutral
D) \[+ve\] field
Correct Answer: B
Solution :
According to\[CFT\], if in a transition metal complex the \[d-\]orbitals of the metal ions shows splitting because of the approach of a ligand as shown below.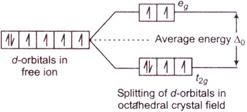 As there is no pairing of electrons and two of its electrons are in high energy orbitals, this is a high spin complex and the ligands causes such high spin states are called weak field ligand.
As there is no pairing of electrons and two of its electrons are in high energy orbitals, this is a high spin complex and the ligands causes such high spin states are called weak field ligand.
You need to login to perform this action.
You will be redirected in
3 sec
