A) propene
B) 2-methylpropene
C) 2-methyl-2-butene
D) 2-methyl-1-butene
Correct Answer: B
Solution :
As formaldehyde has one carbon atom and acetone has three carbon atoms, thus, the alkene must have four carbon atoms, \[i.e.,\] \[2-\]methyl propene.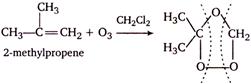 \[\underset{(reductive\,\,cleavage)}{\mathop{\xrightarrow[-ZnO]{Zn/{{H}_{2}}O}}}\,\underset{acetone}{\mathop{C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ || \end{smallmatrix}}{\mathop{C}}\,=O}}\,+\underset{formaldehyde}{\mathop{O=\overset{\begin{smallmatrix} H \\ || \end{smallmatrix}}{\mathop{C}}\,-H}}\,\]
\[\underset{(reductive\,\,cleavage)}{\mathop{\xrightarrow[-ZnO]{Zn/{{H}_{2}}O}}}\,\underset{acetone}{\mathop{C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ || \end{smallmatrix}}{\mathop{C}}\,=O}}\,+\underset{formaldehyde}{\mathop{O=\overset{\begin{smallmatrix} H \\ || \end{smallmatrix}}{\mathop{C}}\,-H}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
