A)
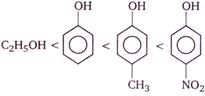
B)
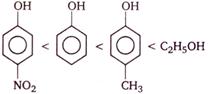
C)
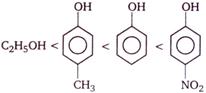
D)
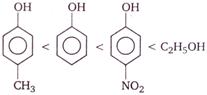
Correct Answer: C
Solution :
Phenols are stronger acids than alcohols, because phenols are considered as resonance hybrid of the following structures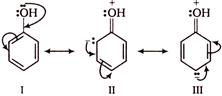
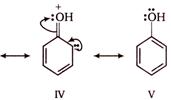 As a result of resonance, the \[O\] atom acquires a partial positive charge. This weakens the \[O-H\] bond and facilitates the release of proton. Thus, phenols are more acidic than alcohols. Now, any electron withdrawing group (\[-N{{O}_{2}}\] group) which stabilize the phenoxide ion by dispersing the negative charge relative to phenol, increases the acidic strength of phenol, whereas any electron donating group (\[-C{{H}_{3}}\]group) which destabilize the phenoxide ion by intensifying the negative charge relative to phenol tend to decrease the acidic strength of phenols. Thus, the correct order of acidity.
As a result of resonance, the \[O\] atom acquires a partial positive charge. This weakens the \[O-H\] bond and facilitates the release of proton. Thus, phenols are more acidic than alcohols. Now, any electron withdrawing group (\[-N{{O}_{2}}\] group) which stabilize the phenoxide ion by dispersing the negative charge relative to phenol, increases the acidic strength of phenol, whereas any electron donating group (\[-C{{H}_{3}}\]group) which destabilize the phenoxide ion by intensifying the negative charge relative to phenol tend to decrease the acidic strength of phenols. Thus, the correct order of acidity. 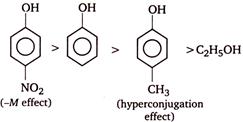
You need to login to perform this action.
You will be redirected in
3 sec
