question_answer 1) For coherent sources, it is essential that their:
A)
amplitudes are equal
done
clear
B)
wavelength are equal
done
clear
C)
frequencies are equal
done
clear
D)
initial phase difference is constant
done
clear
View Answer play_arrow
question_answer 2) In Youngs double slit experiment the intensities of dark and bright lines are 1 and 4 respectively, then the amplitudes of the source are in the ratio:
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[3:1\]
done
clear
D)
\[1:3\]
done
clear
View Answer play_arrow
question_answer 3) The values of voltage and frequency of electric supply for domestic use in India are:
A)
\[\text{220}\,\text{V}\,\text{(r}\text{.m}\text{.s),}\,\text{60}\,\text{Hz}\]
done
clear
B)
\[\text{220}\,\text{V}\,\text{(r}\text{.m}\text{.s),}\,5\text{0}\,\text{Hz}\]
done
clear
C)
\[\text{220}\,\text{V}\,\text{(r}\text{.m}\text{.s),}\,6\text{0}\,\text{Hz}\]
done
clear
D)
\[\text{220}\,\text{V}\,\text{(r}\text{.m}\text{.s),}\,5\text{0}\,\text{Hz}\]
done
clear
View Answer play_arrow
question_answer 4) In bringing an \[\alpha \] particle from rest from a position where electric potential is 70 volt to another position where the potential is 50 volt, the K.E. will be:
A)
\[40eV\]
done
clear
B)
\[40keV\]
done
clear
C)
\[40MeV\]
done
clear
D)
\[0eV\]
done
clear
View Answer play_arrow
question_answer 5) If the length of a so no meter wire is halved, the resonance frequency will become:
A)
three times
done
clear
B)
halved
done
clear
C)
four times
done
clear
D)
double
done
clear
View Answer play_arrow
question_answer 6) The order of pressure in coolidge rube is:
A)
zero mm of \[Hg\]
done
clear
B)
\[{{10}^{-6}}mm\]of \[Hg\]
done
clear
C)
\[{{10}^{-3}}mm\]of \[Hg\]
done
clear
D)
\[0.1mm\]of \[Hg\]
done
clear
View Answer play_arrow
question_answer 7) The radius of fourth half period zone at a point 4 m away from a plane wave front of wavelength \[6400\overset{\text{o}}{\mathop{\text{A}}}\,\]is:
A)
\[32\times {{10}^{-4}}m\]
done
clear
B)
\[30\times {{10}^{-4}}cm\]
done
clear
C)
\[33\times {{10}^{-4}}mm\]
done
clear
D)
\[16\times {{10}^{-3}}m\]
done
clear
View Answer play_arrow
question_answer 8) Two waves represented by \[{{y}_{1}}=a\,\sin \,\left( \omega t+\frac{\pi }{6} \right),{{y}_{2}}=a\,\cos \,\omega t,\] the resultant amplitude will be:
A)
\[a\]
done
clear
B)
\[a\sqrt{2}\]
done
clear
C)
\[a\sqrt{3}\]
done
clear
D)
\[2a\]
done
clear
View Answer play_arrow
question_answer 9) The correct relation between paramagnetic suspectibility X and temperature T is:
A)
\[X=\frac{C}{T}\]
done
clear
B)
\[X=CT\]
done
clear
C)
\[X=\frac{T}{C}\]
done
clear
D)
\[X=C{{T}^{2}}\]
done
clear
View Answer play_arrow
question_answer 10) Moseleys law is:
A)
\[v=a\,(z-\sigma )\]
done
clear
B)
\[v\frac{a}{z-\sigma }\]
done
clear
C)
\[v=a{{(z-\sigma )}^{2}}\]
done
clear
D)
\[v=a\sqrt{z-\sigma }\]
done
clear
View Answer play_arrow
question_answer 11) A person will get more quantity of matter in 1 kg weight at:
A)
poles
done
clear
B)
moon
done
clear
C)
equator
done
clear
D)
artificial satellite
done
clear
View Answer play_arrow
question_answer 12) An elastic ball is released from height of 100 m, and on colliding with earth its 20% energy is dissipated, the height to which ball rebounds:
A)
\[80m\]
done
clear
B)
\[40\text{ }m\]
done
clear
C)
\[60\,m\]
done
clear
D)
\[20\,m\]
done
clear
View Answer play_arrow
question_answer 13) Two identical soap bubbles of radius R are touching each other. The radius of curvature of the common surface is:
A)
infinite
done
clear
B)
R
done
clear
C)
R/2
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 14) Angular velocity of minute hand of a clock is:
A)
\[\frac{\pi }{21600}rad/\sec \]
done
clear
B)
\[\frac{\pi }{12}rad/\sec \]
done
clear
C)
\[\frac{\pi }{3600}rad/\sec \]
done
clear
D)
\[\frac{\pi }{1800}rad/\sec \]
done
clear
View Answer play_arrow
question_answer 15) The presence of atmosphere on a planet means:
A)
\[{{C}_{rms}}<<{{\upsilon }_{e}}\]
done
clear
B)
\[{{C}_{rms}}>{{\upsilon }_{e}}\]
done
clear
C)
The mass of density of planet is small
done
clear
D)
\[{{C}_{rms}}=0\]
done
clear
View Answer play_arrow
question_answer 16) Positive rays are the beam of:
A)
positrons
done
clear
B)
protons
done
clear
C)
\[\alpha \] particles
done
clear
D)
positive ions
done
clear
View Answer play_arrow
question_answer 17) In space, in zero gravity position, when a liquid is heated, then transfer of heat will be in the form :
A)
conduction
done
clear
B)
convection
done
clear
C)
radiation
done
clear
D)
in zero gravity position, liquid cannot be heated
done
clear
View Answer play_arrow
question_answer 18) For L-C-R series A.C. circuit, in resonating conditions which is true?
A)
Minimum current
done
clear
B)
Minimum impendence
done
clear
C)
Power loss minimum
done
clear
D)
Minimum power factor
done
clear
View Answer play_arrow
question_answer 19) If the refracting angle of a bi-prism is increased, what shall be its effect on fringe pattern?
A)
Fringes will come closer
done
clear
B)
Fringes pattern will not be obtained
done
clear
C)
Fringes will not be affected
done
clear
D)
Fringes will get distorted
done
clear
View Answer play_arrow
question_answer 20) The value of\[\frac{{{f}_{2}}}{{{f}_{1}}}\]for zone plate is:
A)
\[\frac{2}{5}\]
done
clear
B)
\[\frac{5}{2}\]
done
clear
C)
\[\frac{3}{9}\]
done
clear
D)
\[\frac{1}{3}\]
done
clear
View Answer play_arrow
question_answer 21) The percentage quantity of a radioactive substance remained after five half-lives will be:
A)
\[31%\]
done
clear
B)
\[3.125%\]
done
clear
C)
\[0.3%\]
done
clear
D)
\[1%\]
done
clear
View Answer play_arrow
question_answer 22) A star is receding from earth with velocity \[{{10}^{6}}m/s\]. If the wavelength of one of its spectral lines is\[5700\overset{o}{\mathop{\text{A}}}\,\], the observed Doppler shift will be:
A)
\[200\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[19\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[20\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.2\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 23) The displacement equation for two waves undergoing superposition are \[{{y}_{1}}=4\,\sin \,\omega t,{{y}_{2}}=3\sin \,(\omega t+\pi /2),\]then resultant amplitude will be:
A)
\[5\,cm\]
done
clear
B)
\[7\,cm\]
done
clear
C)
\[1\,cm\]
done
clear
D)
\[0\,cm\]
done
clear
View Answer play_arrow
question_answer 24) The magnetic moment of a magnetic wire of length L is M. If the wire is bent in form of a semicircle its magnetic moment becomes:
A)
\[\frac{2M}{\pi }\]
done
clear
B)
\[\frac{M}{\pi }\]
done
clear
C)
\[M\]
done
clear
D)
\[M\pi \]
done
clear
View Answer play_arrow
question_answer 25) The isotopes used commonly for treatment of the cancer is:
A)
\[{{K}^{40}}\]
done
clear
B)
\[C{{o}^{60}}\]
done
clear
C)
\[S{{r}^{90}}\]
done
clear
D)
\[{{I}^{131}}\]
done
clear
View Answer play_arrow
question_answer 26) 1 volt is equivalent to:
A)
1 eV
done
clear
B)
\[\frac{joule}{coulomb}\]
done
clear
C)
joule-coulomb
done
clear
D)
\[\frac{erg}{coulomb}\]
done
clear
View Answer play_arrow
question_answer 27) Two sirens 1 km apart are producing sound by \[330\,Hz\]an observer is coming towards a siren from another siren, then apparent frequency will be:
A)
\[8\,Hz\]
done
clear
B)
\[4\,Hz\]
done
clear
C)
\[6\,Hz\]
done
clear
D)
\[1\,Hz\]
done
clear
View Answer play_arrow
question_answer 28) If the distance between a point source of light and a screen is made one third, then relation between intensity of light\[\text{(I)}\] and initial intensity \[\text{(}{{\text{I}}_{0}}\text{)}\]will be:
A)
\[\text{I=}\frac{{{\text{I}}_{\text{0}}}}{\text{3}}\]
done
clear
B)
\[\text{I=}\frac{{{\text{I}}_{\text{0}}}}{9}\]
done
clear
C)
\[\text{I=}{{\text{I}}_{\text{0}}}\]
done
clear
D)
\[\text{I=9}{{\text{I}}_{\text{0}}}\]
done
clear
View Answer play_arrow
question_answer 29) Wavelength of X-ray is of the order:
A)
\[\text{1}{{\text{0}}^{\text{-10}}}\,\text{m}\]
done
clear
B)
\[\text{1}{{\text{0}}^{\text{-10}}}\,\text{cm}\]
done
clear
C)
\[\text{1}{{\text{0}}^{\text{10}}}\,\text{m}\]
done
clear
D)
\[\text{1}{{\text{0}}^{\text{10}}}\,c\text{m}\]
done
clear
View Answer play_arrow
question_answer 30) A chock coil is connected with a fluorescent tube as to:
A)
produce high voltage first, then low current
done
clear
B)
produce high current first, then high voltage.
done
clear
C)
produce high current first, then low voltage.
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 31) Two waves represented as \[x=a\,\sin (\omega t+\pi /6),\,x=a\,\cos \varepsilon t,\] then resultant phase difference between them is:
A)
\[\pi /\text{3}\]
done
clear
B)
\[\pi /\text{6}\]
done
clear
C)
\[\pi /\text{2}\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 32) The intensity of central fringe in a fringe pattern obtained from two slits of equal widths is\[I.\] If one of these slits is closed the intensity at the same place becomes \[{{I}_{0.}}\] The correct relation between \[I\] and \[{{I}_{0.}}\] is:
A)
\[I=4{{I}_{0}}\]
done
clear
B)
\[I=2{{I}_{0}}\]
done
clear
C)
\[I={{I}_{0}}\]
done
clear
D)
\[I=\frac{{{I}_{0}}}{2}\]
done
clear
View Answer play_arrow
question_answer 33) Coherent waves are obtained by method of division of amplitude in which of the following experiments?
A)
Youngs double slit
done
clear
B)
Fresnels biprism
done
clear
C)
Michelson Morley
done
clear
D)
Lyods mirror
done
clear
View Answer play_arrow
question_answer 34) An astronaut is revolving in a circular orbit around the earth at a height 120 km above the surface, then gently drops a pen, then the pen:
A)
will move towards the moon
done
clear
B)
will move down straight towards earth
done
clear
C)
will move with satellite in same direction
done
clear
D)
will move opposite to direction of satellite
done
clear
View Answer play_arrow
question_answer 35) The amplification factor of a triode is 20 and trans conductance is 3 mili mho. If the load resistance is\[3\times {{10}^{4}}\,\Omega ,\] then voltage gain of triode:
A)
\[16.36\]
done
clear
B)
\[28\]
done
clear
C)
\[78\]
done
clear
D)
\[108\]
done
clear
View Answer play_arrow
question_answer 36) The charge is quantized, this is shown by:
A)
Davisson-Germers experiment.
done
clear
B)
Crompton scattering experiment.
done
clear
C)
Milikans oil drop experiment.
done
clear
D)
Raman effect
done
clear
View Answer play_arrow
question_answer 37) The peak value of an A.C. is \[2\sqrt{2}\] A, its rms value will be:
A)
\[\text{1A}\]
done
clear
B)
\[\text{2A}\]
done
clear
C)
\[\text{4A}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) At a frequency more than the resonance frequency, the nature of an anti resonance circuit is:
A)
resistive
done
clear
B)
capacitive
done
clear
C)
inductive
done
clear
D)
all above
done
clear
View Answer play_arrow
question_answer 39) The constant quantity for equal volumes of two gases at same pressure and temperature is:
A)
number of molecules
done
clear
B)
average kinetic energy
done
clear
C)
RMS velocity
done
clear
D)
mean free path
done
clear
View Answer play_arrow
question_answer 40) The meniscus of mercury in a capillary is:
A)
concave
done
clear
B)
convex
done
clear
C)
plane
done
clear
D)
circular
done
clear
View Answer play_arrow
question_answer 41) The moment of inertia of a thin rod of mass M and length L about an axis through one of its ends and perpendiculars length is:
A)
\[\frac{M{{L}^{2}}}{3}\]
done
clear
B)
\[\frac{M{{L}^{2}}}{12}\]
done
clear
C)
\[\frac{M{{L}^{2}}}{2}\]
done
clear
D)
\[\frac{M{{L}^{2}}}{13}\]
done
clear
View Answer play_arrow
question_answer 42) The quantity conserved for earth moving round the sun is:
A)
angular momentum
done
clear
B)
linear momentum
done
clear
C)
rational K.E.
done
clear
D)
kinetic energy
done
clear
View Answer play_arrow
question_answer 43) For an elastic collision:
A)
both kinetic energy and momentum are conserved
done
clear
B)
both kinetic energy and momentum are not conserved
done
clear
C)
either kinetic energy or momentum do not conserved
done
clear
D)
momentum is conserved but not kinetic energy
done
clear
View Answer play_arrow
question_answer 44) A system does 50 J of work under adiabatic condition. In this process:
A)
temperature of system will increase
done
clear
B)
temperature of system will remain same
done
clear
C)
internal energy of system will increase
done
clear
D)
internal energy of system will decrease
done
clear
View Answer play_arrow
question_answer 45) At certain instance the displacement and acceleration of a harmonic oscillator are respectively \[0.02\text{ }m\]and \[2m/{{s}^{2}},\] its angular speed at this instant is (in rad /s):
A)
\[100\]
done
clear
B)
\[10\]
done
clear
C)
\[1\]
done
clear
D)
\[0.1\]
done
clear
View Answer play_arrow
question_answer 46)
An ideal monoatomic gas is taken along the path LMNO as shown in figure, work done during the cycle will be:
A)
PV
done
clear
B)
2 PV
done
clear
C)
3 PV
done
clear
D)
4 PV
done
clear
View Answer play_arrow
question_answer 47) The origin of Fraunhauffer rays is:
A)
reflection of radiations by chromosphere.
done
clear
B)
absorption of raditions by chromosphere.
done
clear
C)
emission of radiations by chromosphere.
done
clear
D)
transmission of radiations by chromosphere.
done
clear
View Answer play_arrow
question_answer 48) If the reactance and resistance of a work coil are \[{{X}_{L}}\] and R respectively, then:
A)
\[{{X}_{L}}=R\]
done
clear
B)
\[{{X}_{L}}>>R\]
done
clear
C)
\[{{X}_{L}}<<R\]
done
clear
D)
\[{{X}_{L}}=\infty \]
done
clear
View Answer play_arrow
question_answer 49)
A pure resistance is connected as shown in the figure, the phase difference between current and e.m.f is:
A)
zero
done
clear
B)
\[\text{/2}\]
done
clear
C)
\[\text{-}\text{/2}\]
done
clear
D)
\[\text{/4}\]
done
clear
View Answer play_arrow
question_answer 50) The path difference, time difference and phase difference between two consecutive half period zones are respectively:
A)
\[\frac{\lambda }{2}\frac{T}{2}\pi \]
done
clear
B)
\[\lambda ,T,\pi \]
done
clear
C)
\[\frac{\lambda }{2}\frac{T}{2}\frac{\pi }{2}\]
done
clear
D)
\[\frac{\lambda }{2}\frac{T}{2}2\pi \]
done
clear
View Answer play_arrow
question_answer 51) To obtain the first minima on screen from a circular aperture the number of zones passing through it should be:
A)
\[4\]
done
clear
B)
\[6\]
done
clear
C)
\[3\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 52) Forbidden energy gap in an intrinsic semiconductor is of the order of:
A)
\[0.1\,eV\]
done
clear
B)
\[1\,eV\]
done
clear
C)
\[5\,eV\]
done
clear
D)
\[10\,eV\]
done
clear
View Answer play_arrow
question_answer 53) The expression relating magnetic induction B magnetizing field H and magnetization intensity \[I\] is:
A)
\[B={{\mu }_{0}}H+I\]
done
clear
B)
\[B={{\mu }_{0}}I+H\]
done
clear
C)
\[B={{\mu }_{0}}(H+I)\]
done
clear
D)
\[B={{\mu }_{0}}(H-I)\]
done
clear
View Answer play_arrow
question_answer 54) With what velocity should a source of sound move away from the observer so that to the observer the frequency of sound may appear to be half of the actual frequency:
A)
\[\frac{\upsilon }{2}\]
done
clear
B)
\[\upsilon 2\]
done
clear
C)
\[\frac{\upsilon }{4}\]
done
clear
D)
\[\upsilon \]
done
clear
View Answer play_arrow
question_answer 55) The intensity of X-ray is controlled in coolidge tube by:
A)
filament current
done
clear
B)
potential difference between cathode and anti cathode
done
clear
C)
both a and b
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 56) The refractive indices of water and glass slab are 4/3 and 5/3 respectively, then critical angle of light entering from glass into water is:
A)
\[{{\sin }^{-1}}\frac{4}{5}\]
done
clear
B)
\[{{\sin }^{-1}}\frac{5}{4}\]
done
clear
C)
\[{{\sin }^{-1}}\frac{1}{2}\]
done
clear
D)
\[{{\sin }^{-1}}\frac{2}{1}\]
done
clear
View Answer play_arrow
question_answer 57) A rocket is receding from earth with a velocity\[0.2c\]. It emits a signal of frequency\[4\times {{10}^{7}}Hz\]. The frequency as observed on earth will be:
A)
\[4\times {{10}^{6}}Hz\]
done
clear
B)
\[3.3\times {{10}^{7}}Hz\]
done
clear
C)
\[3\times {{10}^{6}}\,Hz\]
done
clear
D)
\[5\times {{10}^{7}}Hz\]
done
clear
View Answer play_arrow
question_answer 58) A normally incident ray reflected at an angle of\[{{90}^{o}}\]. The value of critical angle is:
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{65}^{o}}\]
done
clear
D)
\[{{43.5}^{o}}\]
done
clear
View Answer play_arrow
question_answer 59) Waves used in sonography is:
A)
micro waves
done
clear
B)
infrared waves
done
clear
C)
sound waves
done
clear
D)
ultrasonic waves
done
clear
View Answer play_arrow
question_answer 60) If the plate resistance and voltage gain of a triode amplifier are \[15k\Omega \]and 50. Then for a load resistance \[100k\Omega \] amplification factor will be:
A)
\[5\]
done
clear
B)
\[50\]
done
clear
C)
\[57.5\]
done
clear
D)
\[75.5\]
done
clear
View Answer play_arrow
question_answer 61) In the space charge limited region for vacuum tube diode for plate voltage 400 and 200 volts. The plate current and \[{{I}_{{{P}_{1}}}}\]and\[{{I}_{{{P}_{2}}}}\] have ratio:
A)
\[1:2\]
done
clear
B)
\[2:1\]
done
clear
C)
\[1:2\sqrt{2}\]
done
clear
D)
\[2\sqrt{2}:1\]
done
clear
View Answer play_arrow
question_answer 62) Hardness of X-rays produced by Coolidge tube depends on :
A)
filament current
done
clear
B)
air pressure in tube
done
clear
C)
target material
done
clear
D)
potential difference between cathode and target
done
clear
View Answer play_arrow
question_answer 63) At some instant two radioactive substance are having amount in ratio: 1. Their half lives are \[12\,hrs\]and \[16\,hrs\], then after 2 days the ratio of their quantities:
A)
\[1:1\]
done
clear
B)
\[2:1\]
done
clear
C)
\[1:2\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 64) The curve showing correct variation of capacitative reactance \[{{X}_{C}}\] with frequency \[f\]is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 65)
\[{{\upsilon }_{m}}-T\] curves for a perfect black body is:
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 66) A rocket is approaching on the moon with a velocity\[\upsilon \]. The astronaut in rocket sends electromagnetic signals of frequency \[\upsilon \] towards the moon and receives the signals after signals being reflected by the moon. The observed change in frequency will be:
A)
\[\frac{Cv}{C-\upsilon }\]
done
clear
B)
\[\frac{Cv}{C-2\upsilon }\]
done
clear
C)
\[\frac{2v\upsilon }{C}\]
done
clear
D)
\[\frac{2Cv}{\upsilon }\]
done
clear
View Answer play_arrow
question_answer 67) A light wave is incident on a plane face with speed v. After reflection its speed becomes:
A)
\[\upsilon \]
done
clear
B)
\[2\upsilon \]
done
clear
C)
\[3\,\upsilon \]
done
clear
D)
\[4\,\upsilon \]
done
clear
View Answer play_arrow
question_answer 68) The half life of a radioactive element is T and \[\lambda \], is its decay constant, then:
A)
\[\lambda T=1\]
done
clear
B)
\[\lambda T=\frac{1}{2}\]
done
clear
C)
\[\lambda T={{\log }_{e}}2\]
done
clear
D)
\[\lambda =-{{\log }_{e}}2T\]
done
clear
View Answer play_arrow
question_answer 69) The internal resistance of a cell does not depend on:
A)
current drawn from cell
done
clear
B)
distance between electrode
done
clear
C)
concentration of electrolyte
done
clear
D)
e.m.f. of the cell
done
clear
View Answer play_arrow
question_answer 70) The range of magnetic permeability of diamagnetic materials is:
A)
\[-1<\mu <-10\]
done
clear
B)
\[1<\mu <10\]
done
clear
C)
\[1<\mu >10\]
done
clear
D)
\[0<\mu <1\]
done
clear
View Answer play_arrow
question_answer 71) The de Broglices wavelength of an a particle accelerated through a voltage V is:
A)
\[\frac{0.287}{\sqrt{V}}\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[\frac{12.27}{\sqrt{V}}\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[\frac{4.34}{\sqrt{V}}\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\frac{0.202}{\sqrt{V}}\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 72) The wavelength of first line of Balmer series is\[6563\overset{\text{o}}{\mathop{\text{A}}}\,\]. The wavelength of first line of Lyman series is:
A)
\[1215.4\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[7500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[600\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 73) The reactance of an inductor at\[{{10}^{4}}Hz\]. is \[{{10}^{4}}\Omega ,\]then its reactance at \[2\times {{10}^{4}}Hz\]is:
A)
\[{{10}^{4}}\Omega \]
done
clear
B)
\[2\times {{10}^{4}}\Omega \]
done
clear
C)
\[3\times {{10}^{4}}\Omega \]
done
clear
D)
\[4\times {{10}^{4}}\Omega \]
done
clear
View Answer play_arrow
question_answer 74) If the specific resistance and area of cross section of a potentiometer wire are \[\rho \] and A and\[I\]is current flowing through it, then potential gradient is:
A)
\[\frac{I\rho }{A}\]
done
clear
B)
\[\frac{I}{A\rho }\]
done
clear
C)
\[\frac{IA}{\rho }\]
done
clear
D)
\[IA\rho \]
done
clear
View Answer play_arrow
question_answer 75) In stationary waves at antinodes:
A)
energy is maximum
done
clear
B)
pressure and change in density is maximum
done
clear
C)
change in strain is maximum
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 76) The ratio of diameters of fourth and nineth half period zone will be:
A)
\[2/3\]
done
clear
B)
\[4/9\]
done
clear
C)
\[1/4\]
done
clear
D)
\[16/81\]
done
clear
View Answer play_arrow
question_answer 77) The induced e.m.f. in a L-R circuit is maximum:
A)
at the time of switching on due to high resistance
done
clear
B)
at the time of switching off due to high resistance
done
clear
C)
at the time of switching off due to low resistance
done
clear
D)
at the time of switching on due to low resistance
done
clear
View Answer play_arrow
question_answer 78) At normal temperature the degree of freedom for a diatomic gas are:
A)
\[7\]
done
clear
B)
\[6\]
done
clear
C)
\[5\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 79) A ball is released from height h above the ground. If the coefficient of restitution is e, up to what height the ball rises after rebounding twice with the ground:
A)
\[\frac{eh}{2}\]
done
clear
B)
\[2eh\]
done
clear
C)
\[eh\]
done
clear
D)
\[{{e}^{4}}h\]
done
clear
View Answer play_arrow
question_answer 80)
A bob of mass M is connected with two springs as shown in figure the time period of horizontal oscillation of M will be (no damping force is present):
A)
\[2\pi \sqrt{\frac{3k}{M}}\]
done
clear
B)
\[2\pi \sqrt{\frac{k}{M}}\]
done
clear
C)
\[2\pi \sqrt{\frac{M}{2k}}\]
done
clear
D)
\[2\pi \sqrt{\frac{M}{3k}}\]
done
clear
View Answer play_arrow
question_answer 81) Expressions for e.m.f- and current in A.C. circuit are \[E=200sin\text{ }314\text{ }t\]volt and \[I=100\,\sin \,\left( 314t+\frac{\pi }{3} \right)A,\] the power factor will be:
A)
\[1/2\]
done
clear
B)
\[1/4\]
done
clear
C)
\[1\]
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 82)
Two solid sphere is each of mass M and radius R/2 are connected with a mass less rod of length 2R. The moment of inertia of the system about an axis through the centre of any sphere and perpendicular to the rod is:
A)
\[\frac{21}{5}M{{R}^{2}}\]
done
clear
B)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
C)
\[\frac{5}{2}M{{R}^{2}}\]
done
clear
D)
\[\frac{5}{21}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 83)
The water rises to a height of 5 cm in a
A)
5 cm
done
clear
B)
less than 5 cm
done
clear
C)
more than 4 cm
done
clear
D)
none of the these
done
clear
View Answer play_arrow
question_answer 84) Ozone layer is important for us as this :
A)
prevents cooling of earth during night.
done
clear
B)
protects us from intrared rays coming from space.
done
clear
C)
protects us from. metaroids coming from space.
done
clear
D)
protects us from ultravoilet rays coming from space.
done
clear
View Answer play_arrow
question_answer 85) If resonance frequency is f and then the capacity is increased 4 times, then new resonance frequency will be:
A)
\[f\text{/2}\]
done
clear
B)
\[2f\]
done
clear
C)
\[f\]
done
clear
D)
\[f\text{/4}\]
done
clear
View Answer play_arrow
question_answer 86) The velocity of sound in air is \[333\text{ }m/s,\] if the fundamental frequency of an open pipe is 333 Hz Then length of the pipe required to produce second over tone is:
A)
\[0.5m\]
done
clear
B)
\[1.0m\]
done
clear
C)
\[1.5m\]
done
clear
D)
\[2m\]
done
clear
View Answer play_arrow
question_answer 87) Arrange the following in increasing order of work:
A)
\[adiabatic>isothermal>isobaric\]
done
clear
B)
\[isobaric>adiabatic>isothermal\]
done
clear
C)
\[adiabatic>isobaric>isothermal\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 88) On placing a mica sheet of thickness t and refractive index\[\mu \] in the path of first ray producing the interference, by what amount the fringe pattern shifts towards:
A)
\[\frac{d}{D}(\mu -1)t\]
done
clear
B)
\[\frac{D}{d}(\mu -1)t\]
done
clear
C)
\[\frac{d}{(\mu -1)D}\]
done
clear
D)
\[\frac{D}{d}(\mu -1)\]
done
clear
View Answer play_arrow
question_answer 89) The fundamental frequency of an open pipe is\[f.\] The pipe is now vertically dipped in water such that it is half immersed. The fundamental frequency of air column is now:
A)
\[\frac{f}{2}\]
done
clear
B)
\[\frac{3f}{4}\]
done
clear
C)
\[f\]
done
clear
D)
\[2f\]
done
clear
View Answer play_arrow
question_answer 90) The ratio of number of turns in secondary and primary coils of a stepup transformer is\[4:1\]. If the current in the primary coil is 4 amp. The current in the secondary coil is:
A)
\[8A\]
done
clear
B)
\[2A\]
done
clear
C)
\[1A\]
done
clear
D)
\[0.5A\]
done
clear
View Answer play_arrow
question_answer 91)
For the given circuit the reading of voltmeter V and ameter A is:
A)
\[800V,\text{ }2\text{ }A\]
done
clear
B)
\[300V,2\text{ }A\]
done
clear
C)
\[220V,2.2A\]
done
clear
D)
\[100V,2A\]
done
clear
View Answer play_arrow
question_answer 92) On increasing the temperature of a gas filled in a close container by \[{{1}^{o}}C\], its pressure increases by 0.4%, initial temperature of the gas is:
A)
\[{{25}^{o}}C\]
done
clear
B)
\[{{250}^{o}}C\]
done
clear
C)
\[{{250}^{o}}K\]
done
clear
D)
\[{{2500}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 93) For free expansion of van der Waals gas, the final temperature is:
A)
less than initial temperature
done
clear
B)
same as initial temperature
done
clear
C)
more than initial temperature
done
clear
D)
more or less than initial temperature
done
clear
View Answer play_arrow
question_answer 94) depending upon nature of gas The mechanical work required to increase the unit surface area of a liquid is termed as its surface tension, when the liquid is under
A)
isothermal condition
done
clear
B)
isobaric condition
done
clear
C)
isomeric condition
done
clear
D)
adiabatic condition
done
clear
View Answer play_arrow
question_answer 95) A sphere of mass m is moving with constant velocity collides with another identical sphere at rest. The ratio of the velocities of spheres after collision will be: (e = coefficient of restitution)
A)
\[\frac{1-e}{1+e}\]
done
clear
B)
\[\frac{e-1}{e+1}\]
done
clear
C)
\[\frac{1+e}{1-e}\]
done
clear
D)
\[\frac{e+1}{e-1}\]
done
clear
View Answer play_arrow
question_answer 96) The moment of inertia of a figure about its axis is same as its moment of inertia about a perpendicular axis, the figure is:
A)
bisc
done
clear
B)
solid cylinder
done
clear
C)
ring
done
clear
D)
spherical shell
done
clear
View Answer play_arrow
question_answer 97) A clock P is based on a simple pendulum and another clock S is based on spring oscillations. Both the clocks run with the same speed on earth. On a planet having density same as earth but twice the radius:
A)
S will move faster than P
done
clear
B)
P will move faster than P
done
clear
C)
their speeds are same as that on earth
done
clear
D)
their speeds are same but different that on earth
done
clear
View Answer play_arrow
question_answer 98) A body is rolling on a horizontal plane such that its rotational kinetic energy is same as its translational kinetic energy. The body is a:
A)
disc
done
clear
B)
sphere
done
clear
C)
cylinder
done
clear
D)
ring
done
clear
View Answer play_arrow
question_answer 99) Two containers of same volume are filled with molecular hydrogen and helium respectively at 1 and 2 atmospheric pressure. If the temperature of both specimen are same, then average velocity \[<{{C}_{H}}>\]of hydrogen molecules will be:
A)
\[<{{C}_{H}}>=\sqrt{2}<{{C}_{He}}>\]
done
clear
B)
\[<{{C}_{H}}>=<{{C}_{He}}>\]
done
clear
C)
\[<{{C}_{H}}>=2<{{C}_{He}}>\]
done
clear
D)
\[<{{C}_{H}}>=\frac{<{{C}_{He}}>}{2}\]
done
clear
View Answer play_arrow
question_answer 100) Who first discovered that white light consists of seven colours?
A)
Huygen
done
clear
B)
Raman
done
clear
C)
Newton
done
clear
D)
Einestein
done
clear
View Answer play_arrow
question_answer 101) The formation of the phenol from the phenolphthalien is called:
A)
Phenolphthalien reaction
done
clear
B)
Reimer-Tiemanns reaction
done
clear
C)
Coupling reaction
done
clear
D)
Kolbes reaction
done
clear
View Answer play_arrow
question_answer 102) The pyrolusite ore contains:
A)
\[Cu\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Zm\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following compounds distillation with concentrated \[{{H}_{2}}S{{O}_{4}}\]gives mesitylene?
A)
Acetyl chloride
done
clear
B)
Acetaldehyde
done
clear
C)
Acetophenone
done
clear
D)
Acetone
done
clear
View Answer play_arrow
question_answer 104) Which of the following statement is false?
A)
The rate of esterification of alcohols is \[{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\]
done
clear
B)
Catalytic oxidation of methane gives methanol
done
clear
C)
All secondary alcohols give iodoform test
done
clear
D)
All methyl ketones give iodoform test.
done
clear
View Answer play_arrow
question_answer 105) Which of the following have tetrahedral shape?
A)
\[S{{F}_{6}}\]
done
clear
B)
\[I{{F}_{7}}\]
done
clear
C)
\[B{{F}_{3}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 106) Which of the following matching is not possible?
A)
James Chedwick - neutron
done
clear
B)
J. J. Thomson - discovery of electron
done
clear
C)
Faraday - discovery of benzene
done
clear
D)
Graham - law of mass action
done
clear
View Answer play_arrow
question_answer 107)
Arrange the following acids in the ascending order of their \[_{p}{{K}_{a}}\] values: (1) formic acid (2) chloroacetic acid (3) acetic acid (4) trichloroacetic acid
A)
\[4,2,1,3~\]
done
clear
B)
\[4,1,2,3\]
done
clear
C)
\[1,2,3,4\]
done
clear
D)
\[4,3,2,1\]
done
clear
View Answer play_arrow
question_answer 108) Which of the following compound react with aluminium ethoxide and formed a sweet smell-quid?
A)
Acetaldehyde
done
clear
B)
Alcohol
done
clear
C)
Acetone
done
clear
D)
Ether
done
clear
View Answer play_arrow
question_answer 109) The mole of \[{{H}^{+}}\] ion is \[{{10}^{-7}}\] in one litre water. What is the degree of ionization?
A)
\[1.8\times {{10}^{-7}}%\]
done
clear
B)
\[1.8\times {{10}^{-9}}%\]
done
clear
C)
\[3.6\times {{10}^{-7}}%\]
done
clear
D)
\[3.6\times {{10}^{-9}}%\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following is react with methyl alcohol and salt of formic acid with dilute base?
A)
Formaldehyde
done
clear
B)
Acetone
done
clear
C)
Alcohol
done
clear
D)
Ether
done
clear
View Answer play_arrow
question_answer 111) The geometries of some complex ions are given as under:
A)
\[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\] tetrahedral
done
clear
B)
\[[Ag{{(N{{H}_{3)}}_{_{2}}]}^{+}}\]- linear
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\] - octahedral
done
clear
D)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\] - square planar
done
clear
View Answer play_arrow
question_answer 112) Which of the following reagents gives nitrogen on reacting with ethylamine?
A)
Benzoyl chloride
done
clear
B)
Acetyl chloride
done
clear
C)
Nitrosyi chloride
done
clear
D)
Carbon disulphide
done
clear
View Answer play_arrow
question_answer 113) Which of the following are correct match?
A)
\[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}-three\,\,C\] atom \[s{{p}^{2-}}\] hybrid
done
clear
B)
\[HC\equiv C-CH=C{{H}_{2}}\]all the four C atom \[s{{p}^{3}}\] hybrid
done
clear
C)
\[{{(C{{H}_{3}})}_{4}}\] all C atom \[s{{p}^{3}}\] hybrid
done
clear
D)
\[{{C}_{6}}{{H}_{6}}\](benzene) all C atoms \[s{{p}^{2}}\]hybrid
done
clear
View Answer play_arrow
question_answer 114) On the ionization the complex \[CoC{{l}_{3}}.3N{{H}_{3}}\] produce:
A)
one \[C{{l}^{-}}\] ion
done
clear
B)
two \[C{{l}^{-}}\] ion
done
clear
C)
any not \[C{{l}^{-}}\] ion
done
clear
D)
three \[C{{l}^{-}}\] ion
done
clear
View Answer play_arrow
question_answer 115) Given as: \[HF+{{H}_{2}}O\xrightarrow{ka}{{H}_{3}}{{O}^{+}}+{{F}^{-}}\] \[{{F}^{-}}+{{H}_{2}}O\xrightarrow{{{k}_{b}}}HF+O{{H}^{-}}\] Which relation is correct in the following?
A)
\[{{K}_{b}}=K\omega \]
done
clear
B)
\[{{K}_{b}}=1/K\omega \]
done
clear
C)
\[{{K}_{a}}\times {{K}_{b}}={{K}_{\omega }}\]
done
clear
D)
\[Ka/{{K}_{b}}=K\omega \]
done
clear
View Answer play_arrow
question_answer 116) The solubility products of two sparingly soluble electrolytes AB and BC are \[1\times {{10}^{-8}}\] and \[1\times {{10}^{-10}}\] respectively. Which of the following statement is correct?
A)
The solubility cannot be compared from these data.
done
clear
B)
Solubility of AB is 10 times more than that of BC.
done
clear
C)
Solubility of BC is 100 times more than that of AB.
done
clear
D)
Solubility of AB is 100 times more than that of BC.
done
clear
View Answer play_arrow
question_answer 117) The density of ice is less than that of water because:
A)
H bonds are not present in ice
done
clear
B)
water is a polar solvent
done
clear
C)
ice has clusters of molecules and its structure is three dimensional
done
clear
D)
ice floats on water
done
clear
View Answer play_arrow
question_answer 118) Which of the following show the chain isomerism?
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-OH\]
done
clear
C)
\[C{{H}_{2}}=CH-C{{H}_{2}}-OH\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-Cl\]
done
clear
View Answer play_arrow
question_answer 119) Which of the following is not a correct match?
A)
\[C{{H}_{3}}CHO+{{I}_{2}}+NaOH\xrightarrow{{}}\]a yellow precipitate
done
clear
B)
\[CHC{{l}_{3}}+HN{{O}_{3}}\to \]a war gas
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH-CaOC{{l}_{2}}\to \]an anaesthetic
done
clear
D)
\[CHC{{l}_{3}}+C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\to \] a solvent
done
clear
View Answer play_arrow
question_answer 120) Which of these differ in the hybridisation?
A)
\[HgC{{l}_{2}}\],
done
clear
B)
\[ZnC{{l}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[C{{F}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 121)
Match list-I with list-II and select the correct set form the sets given below: List-I List-II (A) \[N{{H}_{2}}CONHN{{O}_{2}}\] (X) Diphenyl urea (B) \[N{{H}_{2}}CONHCON{{H}_{2}}\] (Y) Nitro urea (C) \[N{{H}_{2}}CONHN{{H}_{2}}\] (Z) Semi carbazide (D) \[{{({{C}_{6}}{{H}_{5}}NH)}_{2}}CO\] (X)Biuret
A)
A-Y B-X C-Z D-X
done
clear
B)
A-X B-Y C-Z D-X
done
clear
C)
A-X B-Z C-Y D-X
done
clear
D)
A-X B-Y C-X D-Z
done
clear
View Answer play_arrow
question_answer 122) The solubility product of \[BaS{{O}_{4}}\]is\[1\times {{10}^{-12}}\]. What will be the concentration of barium ions in its saturation solution?
A)
\[1\times {{10}^{6}}\]
done
clear
B)
\[1\times {{10}^{-6}}\]
done
clear
C)
\[1\times {{10}^{12}}\]
done
clear
D)
\[1\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
question_answer 123) For an equilibrium reaction, \[{{H}_{2}}+{{I}_{2}}2HI\] the value of \[Kc=49\]at a \[{{444}^{o}}C\] temperature. For the equilibrium reaction, \[HI\frac{1}{2}{{H}_{2}}+\frac{1}{2}{{I}_{2}}\]the value of \[{{K}_{c}}\] at this temperature is:
A)
\[49\]
done
clear
B)
\[0.143\]
done
clear
C)
\[Erors\,0.02\]
done
clear
D)
\[7\]
done
clear
View Answer play_arrow
question_answer 124) The solubility \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\] is s mole. What is the solubility product of \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\]?
A)
\[8{{s}^{3}}\]
done
clear
B)
\[9{{s}^{2}}\]
done
clear
C)
\[108{{s}^{5}}\]
done
clear
D)
\[72{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 125) In which of the following pairs of oxidation states of sulphur and chromium are same?
A)
\[S{{O}_{2}},Cr{{O}_{4}}^{-2}\]
done
clear
B)
\[S{{O}_{2}},Cr{{O}_{7}}^{-2}\]
done
clear
C)
\[S{{O}_{3}},Cr{{O}_{4}}^{-2}\]
done
clear
D)
\[S{{O}_{3}}^{-2},Cr{{O}_{4}}^{-2}\]
done
clear
View Answer play_arrow
question_answer 126) Fused sodium chloride can conduct electricity because of:
A)
free ions
done
clear
B)
free molecules
done
clear
C)
free electrons
done
clear
D)
free atoms
done
clear
View Answer play_arrow
question_answer 127) \[5moles\]of sulphur bum in oxygen. What does found the quantity of\[S{{O}_{2}}\]?
A)
\[640g\]
done
clear
B)
\[80g\]
done
clear
C)
\[160g\]
done
clear
D)
\[320g\]
done
clear
View Answer play_arrow
question_answer 128) If the density of silver is \[10.8g\text{ }c{{m}^{-8}}\]then which of the following volume of an atom of silver?
A)
\[1.66\times {{10}^{-23}}c{{m}^{3}}\]
done
clear
B)
\[1.66\times {{10}^{-20}}c{{m}^{3}}\]
done
clear
C)
\[1.66\times {{10}^{-22}}c{{m}^{3}}\]
done
clear
D)
\[1.66\times {{10}^{-18}}c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 129) Which of the following compound have 1 oxidation number of chlorine?
A)
\[C{{l}_{2}}O\]
done
clear
B)
\[ICl\]
done
clear
C)
\[HCl{{O}_{4}}\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 130) \[Starch\xrightarrow{A}maltose\xrightarrow{B}\text{glucose}\]\[\xrightarrow{C}ethyl\,alcohol\]. In this reaction sequence A, B and C are respectively:
A)
zymase, maltase, invertase
done
clear
B)
diastose, maltase, zymase
done
clear
C)
invertase, zymase, maltase
done
clear
D)
invertase, maltase, zymase
done
clear
View Answer play_arrow
question_answer 131) In reaction equilibrium: \[{{N}_{3}}+3{{H}_{2}}\xrightarrow{{}}2N{{H}_{3}}+22\text{ }kcal\] the highest yield of ammonia is favoured by:
A)
increase in temperature
done
clear
B)
decrease in pressure
done
clear
C)
increase in pressure
done
clear
D)
addition of ammonia
done
clear
View Answer play_arrow
question_answer 132) The pair of iso-electronic species in the following is:
A)
\[N{{O}_{3}}^{-}\text{, }HC{{O}_{3}}\]
done
clear
B)
\[{{N}_{2}},CO\]
done
clear
C)
\[N{{O}_{3}}^{-},C{{O}_{3}}^{-2}\]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 133) Which of the following is not a correct match? (1) Methane nitrile \[C{{H}_{3}}CN\] (2) Iso butyric acid \[{{(C{{H}_{3}})}_{3}}C-COOH\] (3) Crotonic acid \[C{{H}_{3}}-CH=CH-COOH\] (4) Acetonitrile \[C{{H}_{3}}-C\equiv N\]
A)
\[1,4\]
done
clear
B)
\[2,3\]
done
clear
C)
\[1,3\]
done
clear
D)
\[2,4\]
done
clear
View Answer play_arrow
question_answer 134) Which of the following test gives blue colour with s-butyl alcohol:
A)
By iodoform test
done
clear
B)
By Victor Mayors test
done
clear
C)
By chloroform test
done
clear
D)
None
done
clear
View Answer play_arrow
question_answer 135) On which of the following addition of the Grignard reagent:
A)
done
clear
B)
\[-C\equiv N\]
done
clear
C)
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 136) Which of the following is not a correct match?
A)
Rock salt- \[NaCl\]
done
clear
B)
Food soda -\[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\]
done
clear
C)
Soda ash - \[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
Caustic soda - \[NaOH\]
done
clear
View Answer play_arrow
question_answer 137) The number of isomers of the compound of molecular formula \[{{C}_{2}}{{H}_{5}}B{{r}_{2}}\] is:
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[2\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 138) The primary amine can be easily tested by its reaction with the following:
A)
alkyl chloride
done
clear
B)
chloride
done
clear
C)
acetyl chloride
done
clear
D)
chloroform and base
done
clear
View Answer play_arrow
question_answer 139) The melting point of \[SnC{{l}_{2}}\]is less than that of \[SnC{{l}_{4}}\]. The reason is:
A)
higher positive charge on \[S{{n}^{4+}}\]
done
clear
B)
higher ionic potential of \[S{{n}^{4+}}\]
done
clear
C)
tetrahedral structure of \[SnC{{l}_{4}}\]
done
clear
D)
size of \[S{{n}^{4+}}\] is smaller than that of \[S{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 140) The LU.P.A.C. name of \[\underset{CH=CH-COOH}{\overset{C{{H}_{2}}-COOH}{\mathop{|}}}\,\]is:
A)
2-pentene-1, 5 di oic acid
done
clear
B)
4-pentene-1, 5 di oic acid
done
clear
C)
both (a) and (b)
done
clear
D)
None
done
clear
View Answer play_arrow
question_answer 141) Nitroso amine \[+\] phenol \[\xrightarrow{conc.\,{{H}_{2}}S{{O}_{4}}}\]red or brown colour \[\xrightarrow{base}\] blue or violet. The name of this reaction is:
A)
Lucas test
done
clear
B)
Coupling reaction
done
clear
C)
Ledrer- Mannase reaction
done
clear
D)
Libermann nitroso reaction
done
clear
View Answer play_arrow
question_answer 142) Which of the following reagent used in the difference between methanol and ethanol?
A)
\[{{I}_{2}}+NaOH\]
done
clear
B)
Schiff reagent
done
clear
C)
Cromic acid
done
clear
D)
Lucas test
done
clear
View Answer play_arrow
question_answer 143) Which of the element has very low ionization potential:
A)
\[F\]
done
clear
B)
\[H\]
done
clear
C)
\[Cl\]
done
clear
D)
\[Fr\]
done
clear
View Answer play_arrow
question_answer 144) Ethene and ethyne can be distinguished by the reaction with:
A)
anhydrous \[ZnC{{l}_{2}}+\] cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
dilute alkaline \[KMn{{O}_{4}}\]
done
clear
C)
iodine and \[NaOH\]
done
clear
D)
ammonical cuprous chloride
done
clear
View Answer play_arrow
question_answer 145) The \[_{p}{{K}_{a}}\] values for the dissociation of carbonic acid, acetic acid and formic acid are respectively \[6.3,4.74,\] and \[3.62\]. Which of the following statement is wrong?
A)
Acetic acid is weakest in all three
done
clear
B)
Formic acid is strongest in all the three
done
clear
C)
Carbonic acid is strongest in all the three
done
clear
D)
Acetic acid is weaker than carbonic acid
done
clear
View Answer play_arrow
question_answer 146) How many \[\sigma \] bond and \[\pi \] bond has this structures? \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\underset{CH-C{{H}_{3}}}{\mathop{\underset{||}{\mathop{C}}\,}}\,H\]
A)
\[5\sigma ,\,1\pi \]
done
clear
B)
\[17\sigma ,\,1\sigma \]
done
clear
C)
\[17\sigma ,\,1\pi \]
done
clear
D)
\[1\sigma ,\,5\pi \]
done
clear
View Answer play_arrow
question_answer 147) In which acid has not \[-COOH\]group?
A)
Lactic acid
done
clear
B)
Succinic acid
done
clear
C)
Carbonic acid
done
clear
D)
Barbituric acid
done
clear
View Answer play_arrow
question_answer 148) The I.U.RA.C. name of acetone is:
A)
2-propanon
done
clear
B)
2-propanal
done
clear
C)
2-butanon
done
clear
D)
2-propanol
done
clear
View Answer play_arrow
question_answer 149) Which of the following statements is correct?
A)
Electron emits energy in the transition from higher energy level to lower energy level
done
clear
B)
Electron emits energy spontaneously in its ground state.
done
clear
C)
Electron cannot fall in the nucleus from third energy level
done
clear
D)
Electron absorbs energy in the transition from lower energy level to higher energy level
done
clear
View Answer play_arrow
question_answer 150) Which of the following compound gives halo form reaction?
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
Both above
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 151) Which of the following expressions is not applicable on the hydrolysis equilibrium? \[N{{H}_{4}}^{+}+{{H}_{2}}ON{{H}_{4}}OH+{{H}^{+}}\]
A)
\[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{b}}}\]
done
clear
B)
\[[{{H}^{+}}]=\sqrt{\frac{{{K}_{\omega }}\times C}{{{K}_{b}}}}\]
done
clear
C)
\[h=\sqrt{\frac{{{K}_{\omega }}}{{{K}_{b}}\times C}}\]
done
clear
D)
\[pH=\frac{1}{2}{{\,}_{p}}{{K}_{a}}(N{{H}_{4}}OH)\]
done
clear
View Answer play_arrow
question_answer 152) A carbonyl compound (X) reacts with \[HCN\] to give a compound (V). The product (Z) obtained by the hydrolysis of compound (Y) shows optical isomerism and also gives iodoform test. Compound (X)is:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 153) Which of the following is not correct?
A)
\[s{{p}^{2}}\]-trigonal planar
done
clear
B)
\[s{{p}^{3}}\]-trigonal bipyramidal
done
clear
C)
\[s{{p}^{3}}\]-trigonal
done
clear
D)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 154) The indicator for the iodine titration is:
A)
phenophthaline
done
clear
B)
galactose
done
clear
C)
cellulose
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 155) \[3A+B2C\] what is K for this system:
A)
\[\frac{[C]}{[3A][B]}\]
done
clear
B)
\[\frac{[C]}{{{[A]}^{3}}\times [B]}\]
done
clear
C)
\[\frac{{{[C]}^{2}}}{{{[A]}^{3}}\times [B]}\]
done
clear
D)
\[\frac{[3A]\times [B]}{[2C]}\]
done
clear
View Answer play_arrow
question_answer 156) Which of the following reaction generally shown by alkenes and alkynes?
A)
Electrophilic addition
done
clear
B)
Nucleophilic addition
done
clear
C)
Free radical substitution
done
clear
D)
Electrophilic substitution
done
clear
View Answer play_arrow
question_answer 157) Which of the following sets is correct?
A)
\[n=3,l=2,\,m=-1,\,s=-\frac{1}{2}\]
done
clear
B)
\[n=3,l=2,\,m=-3,\,s=+\frac{1}{2}\]
done
clear
C)
\[n=3,l=3,\,m=0,\,s=+\frac{1}{2}\]
done
clear
D)
\[n=3,l=3,\,m=-3,\,s=-\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 158) The product of the reaction of hydrazine with ethyl acetate:
A)
\[C{{H}_{3}}COONHN{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}COHN-NHCOC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CONHN{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 159) The thin layer of which of the following is deposited in the galvanized iron?
A)
White lead
done
clear
B)
Zinc
done
clear
C)
Tin
done
clear
D)
Aluminium
done
clear
View Answer play_arrow
question_answer 160) When the ketone react with hydrogen at \[{{160}^{o}}C\]and formed alkane this reaction is known as:
A)
Witting reaction
done
clear
B)
Wolff Kishner reaction
done
clear
C)
Jijal method
done
clear
D)
Wurtz-fitting reaction
done
clear
View Answer play_arrow
question_answer 161) The energy of sub-shells increases with the:
A)
increase of spin quantum number
done
clear
B)
increase of azimuthal quantum no.
done
clear
C)
increase of principal quantum no.
done
clear
D)
increase of principal and azimuthal quantum number both
done
clear
View Answer play_arrow
question_answer 162) Which one of the following reaction is different than the others?
A)
\[Mg\xrightarrow{{}}M{{g}^{++}}+2{{e}^{-}}\]
done
clear
B)
\[F{{e}^{2+}}\xrightarrow{{}}F{{e}^{3+}}+3{{e}^{-}}\]
done
clear
C)
\[S+2{{e}^{-}}\xrightarrow{{}}{{S}^{-\,\,-}}\]
done
clear
D)
\[S{{n}^{4}}+2e\xrightarrow{{}}S{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 163) Which of the following shows diagonal relation?
A)
Element of first period
done
clear
B)
Element of second period
done
clear
C)
Element of third period
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 164) The I.U.P.A.C name of this compound \[\underset{\begin{smallmatrix} | \\ CN \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}-\underset{\begin{smallmatrix} | \\ CN \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ CN \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\]
A)
1, 2, 3 tricyano propane
done
clear
B)
3-cyano-l, 5 pentane dinitrile
done
clear
C)
1, 2, 3-cyano propane
done
clear
D)
1, 2, 3-propane trinitrite
done
clear
View Answer play_arrow
question_answer 165) Which of the following compounds is formed by the reaction of propyne with \[{{H}_{2}}S{{O}_{4}}\] and mercuric ions?
A)
Propanal
done
clear
B)
2-propanal
done
clear
C)
Propanone
done
clear
D)
Propane diol
done
clear
View Answer play_arrow
question_answer 166) Which of the following statements is false?
A)
1-alkynes do not show geometrical isomerism
done
clear
B)
\[\alpha \]-butylene and isopropyl alcohol are not position isomers
done
clear
C)
Alcohols and ethers with same molecular weight are functional group isomers
done
clear
D)
\[\alpha \]-butylene is a chain isomers of isobutylene
done
clear
View Answer play_arrow
question_answer 167) Acetaldehyde is obtained by the ozonolysis of the following alkene:
A)
propene
done
clear
B)
isobutylene
done
clear
C)
2-butene
done
clear
D)
1-butene
done
clear
View Answer play_arrow
question_answer 168) Metal exists in the following form after calcination:
A)
as sulphide
done
clear
B)
as carbonate
done
clear
C)
hydrated oxide
done
clear
D)
an oxide
done
clear
View Answer play_arrow
question_answer 169) Decreasing order of an electron affinity of halogen is:
A)
\[Cl>Br>F>I~\]
done
clear
B)
\[Cl>F>Br>I\]
done
clear
C)
\[Br>Cl>F>I~\]
done
clear
D)
\[F>Cl>Br>I\]
done
clear
View Answer play_arrow
question_answer 170) The reaction of ether with cold \[HI\] is called:
A)
Zerewiriknoff method
done
clear
B)
Ziesels method
done
clear
C)
Williamsons synthesis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 171)
A)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\] and : \[CHCl\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\]-and: \[C{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\] and: \[CC{{l}_{3}}\]
done
clear
D)
phenoxide ion and dichloro carbine
done
clear
View Answer play_arrow
question_answer 172) The gas produced by the reaction acetamide with aqueous alkali is:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[NO\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 173) Methyl orange shows the following colour in alkaline medium:
A)
yellow
done
clear
B)
colourless
done
clear
C)
orange
done
clear
D)
red
done
clear
View Answer play_arrow
question_answer 174) All are polymer except:
A)
teflon
done
clear
B)
orlon
done
clear
C)
nylon
done
clear
D)
phorone
done
clear
View Answer play_arrow
question_answer 175) The correct sequence of the stability of carbocation is:
A)
\[{{3}^{o}}<{{2}^{o}}>1{}^\circ >C{{H}_{3}}\]
done
clear
B)
\[{{3}^{o}}<\text{ }{{2}^{o}}\text{ }{{1}^{o}}<C{{H}_{3}}\]
done
clear
C)
\[{{3}^{o}}<{{2}^{o}}>{{1}^{o}}<C{{H}_{3}}\]
done
clear
D)
\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}>C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 176) The degree of ionization in weak electrolyte is directly proportional to the following:
A)
volume (V)
done
clear
B)
concentration (C)
done
clear
C)
both above
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 177) The density of Na greater than \[K\]. The reason is:
A)
there are eight electrons in third shell of \[K\]
done
clear
B)
the size of \[Na\] is shorter than \[K\]
done
clear
C)
ionization potential of \[Na\] is lesser than \[K\]
done
clear
D)
Atomic wt. of \[K\] is more than of \[Na\]
done
clear
View Answer play_arrow
question_answer 178) Vinegar is:
A)
8-10% acetic acid
done
clear
B)
6-10% ethyl alcohol
done
clear
C)
10% formic acid
done
clear
D)
glacial acetic acid
done
clear
View Answer play_arrow
question_answer 179) Which of following product obtained by the reaction between nitrobenzene and \[Zn/N{{H}_{4}}C{{l}^{-}}\]?
A)
Ammo phenol
done
clear
B)
Nitroso benzene
done
clear
C)
Phenyl hydroxyl amine
done
clear
D)
Azobenzene
done
clear
View Answer play_arrow
question_answer 180) Water gas is a mixture of:
A)
\[1mole\,\,CO+1mole\,{{H}_{2}}\]
done
clear
B)
\[1\text{ }mole\text{ }CO+\text{ }1\text{ }mole\]water vapour
done
clear
C)
\[1\,\,moleCO+1\,mole\,{{H}_{2}}\]
done
clear
D)
\[2\,mole\,CO+1mole\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 181) \[Ar-CON{{H}_{2}}\xrightarrow{B{{r}_{2}}+KOH}ArN{{H}_{2}}\] The above reaction is:
A)
Hoffmann-martius rearrangement
done
clear
B)
Haffmann carbylamine reaction
done
clear
C)
Haffmann mustard oil reaction
done
clear
D)
Haffmann bromide reaction
done
clear
View Answer play_arrow
question_answer 182) \[Aniline\xrightarrow[{{150}^{o}}C]{conc.\,\,{{H}_{2}}S{{O}_{4}}}\text{ }A\]. The product A is:
A)
aniline sulphate
done
clear
B)
sulphonic acid
done
clear
C)
sulphanilic acid
done
clear
D)
o-amino benzene sulphonic acid
done
clear
View Answer play_arrow
question_answer 183) \[C{{H}_{3}}MgBr+A\xrightarrow{{}}B\xrightarrow{{{H}_{2}}O/{{H}^{+}}}\] \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\] In the above reaction sequence A is:
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CO\]
done
clear
C)
\[{{(C{{H}_{2}})}_{2}}O\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 184) If pH = 8 then what is the value of \[[{{H}^{+}}]\] and which type solution found:
A)
\[{{10}^{-8}}\]basic
done
clear
B)
\[0.00001\]basic
done
clear
C)
\[{{10}^{-8}}\]acidic
done
clear
D)
\[0.00001\]acidic
done
clear
View Answer play_arrow
question_answer 185) The set of transition ions which have same magnetic moment is:
A)
\[C{{u}^{+2}},\,F{{e}^{+2}}\]
done
clear
B)
\[M{{n}^{+2}},T{{i}^{+2}}\]
done
clear
C)
\[M{{n}^{+2}},F{{e}^{+3}}\]
done
clear
D)
\[C{{u}^{+2}},F{{e}^{+3}}\]
done
clear
View Answer play_arrow
question_answer 186) The wavelength of all the spectral lines of H atom can be given by the following expression:
A)
\[\frac{1}{\lambda }=R\left( \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right)\]
done
clear
B)
\[\frac{1}{\lambda }=R\left( \frac{1}{{{n}_{2}}^{2}}-\frac{1}{{{n}_{1}}^{2}} \right)\]
done
clear
C)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{1}}}-\frac{1}{{{n}_{2}}} \right)}^{2}}\]
done
clear
D)
\[\frac{1}{\lambda }=R{{\left( \frac{1}{{{n}_{2}}}-\frac{1}{{{n}_{1}}} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 187) Which is the main constituent of Nilam?
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[A{{g}_{2}}O\]
done
clear
C)
\[C{{u}_{2}}O\]
done
clear
D)
\[MnO\]
done
clear
View Answer play_arrow
question_answer 188) The formula of potash alum is:
A)
\[{{K}_{2}}S{{O}_{4}}.A{{l}_{2}}{{(S{{O}_{4}})}_{3}}.24{{H}_{2}}O\]
done
clear
B)
\[{{K}_{3}}[CO{{(N{{O}_{2}})}_{6}}]\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}.C{{r}_{2}}{{(S{{O}_{4}})}_{3}}.24{{H}_{2}}O\]
done
clear
D)
\[KH{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 189) The relation between solubility and solubility product is:
A)
solubility product \[={{(solubility)}^{2}}\]
done
clear
B)
solubility product = \[=\sqrt{solubility}\]
done
clear
C)
solubility product \[=1/solubility\]
done
clear
D)
solubility product = solubility
done
clear
View Answer play_arrow
question_answer 190) The oxidation number of nitrogen in \[N{{H}_{4}}\]and \[N{{O}_{3}}\]is:
A)
\[+3\]
done
clear
B)
\[-3\] and \[+5\]
done
clear
C)
\[+5\]
done
clear
D)
\[+3\]and \[-5\]
done
clear
View Answer play_arrow
question_answer 191) In ail equilibrium reaction \[A\xrightarrow{{}}2B+C\]the degree of dissociation of A is an initially one mole of a is taken then number of total moles at equilibrium:
A)
\[1-2a\]
done
clear
B)
\[1-a\]
done
clear
C)
\[1+2a\]
done
clear
D)
\[1+a\]
done
clear
View Answer play_arrow
question_answer 192) Benzene reacts with chlorine in the presence of UV-light to give:
A)
1, 3, 5 trichlorobenzene
done
clear
B)
chlorobenzene
done
clear
C)
hexachloro cyclohexane
done
clear
D)
hexa chlorobenzene
done
clear
View Answer play_arrow
question_answer 193) Which of the following is not a correct match?
A)
Fools gold \[(Fe{{S}_{2}})\]
done
clear
B)
Philosphers wool \[ZnO\]
done
clear
C)
Calomel \[H{{g}_{2}}C{{l}_{2}}\]
done
clear
D)
Lunar caustic \[AgCl\]
done
clear
View Answer play_arrow
question_answer 194) In which of the following the dipole moment is zero?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 195) The electronic configuration of \[Na\] is\[2,8,1\]. How many proton and electron of it?
A)
\[11,11\]
done
clear
B)
\[2,3\]
done
clear
C)
\[5,2\]
done
clear
D)
\[3,5\]
done
clear
View Answer play_arrow
question_answer 196) The uses of carbon tetra chloride is:
A)
industrial solvent and fire extinguisher
done
clear
B)
Coolent and fire extinguisher
done
clear
C)
Anaesthetic and Antiseptics
done
clear
D)
Antiseptics and laboratory solvent
done
clear
View Answer play_arrow
question_answer 197) Which of the following is colourless and smellness?
A)
\[C{{H}_{3}}NC\]
done
clear
B)
\[C{{H}_{3}}NC\]
done
clear
C)
\[C{{H}_{3}}I\]
done
clear
D)
\[C{{F}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 198) \[X+Y\xrightarrow{\Delta }HCOOH\xrightarrow{{{H}_{2}}S{{O}_{4}}/\Delta },L+M\] X, V, L, M are respectively:
A)
\[C{{O}_{2}},{{H}_{2}}O,C{{O}_{2}},{{H}_{2}}\]
done
clear
B)
\[CO,{{H}_{2}}O,CO,{{H}_{2}}O\]
done
clear
C)
\[CO,{{H}_{2}}O,C{{O}_{2}},{{H}_{2}}O\]
done
clear
D)
\[C{{O}_{2}},{{H}_{2}}O,CO,{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 199) \[NaCl\] is a compound:
A)
covalent
done
clear
B)
co-ordinate valent
done
clear
C)
electro valent
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 200) The Bronsted base is: In this reaction, \[H{{C}_{2}}{{O}_{4}}+P{{O}_{4}}^{3-}HP{{O}_{4}}^{2-}+{{C}_{2}}{{O}_{4}}^{2-}\]
A)
\[H{{C}_{2}}{{O}_{4}}^{-},HP{{O}_{4}}^{2-}\]
done
clear
B)
\[P{{O}_{4}}^{3-},HP{{O}_{4}}^{2-}\]
done
clear
C)
\[P{{O}_{4}}^{3-},{{C}_{2}}{{O}_{4}}^{2-}\]
done
clear
D)
\[H{{C}_{2}}{{O}_{4}}^{-},{{C}_{2}}{{O}_{4}}^{2-}\]
done
clear
View Answer play_arrow
question_answer 201) The association between Hydra and too zoo chlorelles are:
A)
commensalism
done
clear
B)
symbiosis
done
clear
C)
parasitism
done
clear
D)
saprophytic
done
clear
View Answer play_arrow
question_answer 202) Which one is Devil fish?
A)
Rhinobates
done
clear
B)
Octopus
done
clear
C)
Sepia
done
clear
D)
Giant squid
done
clear
View Answer play_arrow
question_answer 203) Pernicious anaemia is caused by deficiency of:
A)
folic acid
done
clear
B)
retinol
done
clear
C)
cyanocobalamine
done
clear
D)
biotin
done
clear
View Answer play_arrow
question_answer 204) Amoeba lack:
A)
centrosome
done
clear
B)
mitochondria
done
clear
C)
ribosome
done
clear
D)
E.R.
done
clear
View Answer play_arrow
question_answer 205) Liver of Rabbit secretes:
A)
cholesterol
done
clear
B)
bile pigment
done
clear
C)
bile salt
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 206) Middle ear protects the internal ear from. high sound waves :
A)
by the reflex action of tenser tympani and stapes muscle
done
clear
B)
by pulling inside malleus and tympanic membrane
done
clear
C)
by pulling outside the stapes and basal plates
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 207) Normally creatine is absent in urine of:
A)
pregnant female
done
clear
B)
healthy man
done
clear
C)
starving man
done
clear
D)
lactating mothers
done
clear
View Answer play_arrow
question_answer 208) What is the function of hydrostatic skeleton?
A)
Locomotion
done
clear
B)
Climbing
done
clear
C)
Looping
done
clear
D)
Somersalt
done
clear
View Answer play_arrow
question_answer 209) E.R. is non-continuous with:
A)
nuclear membrane
done
clear
B)
plasm a lemma
done
clear
C)
golgibody
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 210) A statement which is false for eyes in resting state?
A)
Eyes are focused at placed objects
done
clear
B)
Hyes are focused at nearly objects
done
clear
C)
Cilliary muscles are relaxed
done
clear
D)
Suspensory ligaments are connected
done
clear
View Answer play_arrow
question_answer 211) Which one is poisonous Lizard?
A)
Flying Lizard
done
clear
B)
Granular Lizard
done
clear
C)
Sand Lizard
done
clear
D)
Bedod Lizard
done
clear
View Answer play_arrow
question_answer 212) Concentration and pressure of CO in the alveoli of lungs in human beings is :
A)
1.0% and 0.7 mm Hg
done
clear
B)
0.4% and 0.4 mm Hg
done
clear
C)
2 to 7% and 0.4 mm Hg
done
clear
D)
0.3 and 0.4 mm Hg
done
clear
View Answer play_arrow
question_answer 213) At which cell stage a blastula can be implanted in uterus in test tube baby development?
A)
16 cell
done
clear
B)
64 cell
done
clear
C)
128 cell
done
clear
D)
32 cell
done
clear
View Answer play_arrow
question_answer 214) Depression in Hydra is responsible for:
A)
death
done
clear
B)
rejuvenation
done
clear
C)
budding
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 215) In RNA sample which one is found:
A)
A = T and C = G
done
clear
B)
A = U and C = G
done
clear
C)
Purine = pyrimidine
done
clear
D)
A # U # C # G
done
clear
View Answer play_arrow
question_answer 216) Which character of inorganic catalyst does not resemble enzymes ?
A)
catalytic property
done
clear
B)
temperature specicity
done
clear
C)
colloidal state
done
clear
D)
reversible reaction
done
clear
View Answer play_arrow
question_answer 217) Which statement is incorrect for Hydra?
A)
Nematocyst present
done
clear
B)
It is of phylum Cnideria
done
clear
C)
Physiological division of labour present
done
clear
D)
They arc hermaphrodite
done
clear
View Answer play_arrow
question_answer 218) One molecule of glucose releases energy :
A)
68600 kcal
done
clear
B)
686000 kcal
done
clear
C)
6860000 kcal
done
clear
D)
786000 kcal
done
clear
View Answer play_arrow
question_answer 219) Which disorder is more common due to protein deficiency?
A)
Paralysis
done
clear
B)
Rickets
done
clear
C)
Osteomalacia
done
clear
D)
Kwashiorkor
done
clear
View Answer play_arrow
question_answer 220) Which of the following pairs is wrong?
A)
Vitamin D-fats
done
clear
B)
Pectin-protein
done
clear
C)
Heparin-polysaccharides
done
clear
D)
Chi tin-carbohydrate
done
clear
View Answer play_arrow
question_answer 221) Which hormone acts opposite to the parathormone?
A)
Corticosteron
done
clear
B)
Thyrocalcitonin
done
clear
C)
Thyroxine
done
clear
D)
Mineral ocorticoids
done
clear
View Answer play_arrow
question_answer 222) In an egg of Frog is divided in a way that one part has animal hemisphere and another part is deficient in that then:
A)
only animal hemisphere region forms embryo
done
clear
B)
both the sites form embryo
done
clear
C)
embryo is not formed from any region
done
clear
D)
animal hemisphere deficient region forms the embryo
done
clear
View Answer play_arrow
question_answer 223) Which of the following is not a part of corpus callosum?
A)
Fornix
done
clear
B)
Crura cerebri
done
clear
C)
Zenue
done
clear
D)
Splengion
done
clear
View Answer play_arrow
question_answer 224) What is not a function of para sympathetic nervous system?
A)
Dilation of eye pupil
done
clear
B)
To decrease the neart beat
done
clear
C)
To dilate the blood vessel
done
clear
D)
To relax anal muscles
done
clear
View Answer play_arrow
question_answer 225) Basic unit of classification is:
A)
series
done
clear
B)
genus
done
clear
C)
class
done
clear
D)
species
done
clear
View Answer play_arrow
question_answer 226) Sequential and regular conversion of zygote into ail animal is known as:
A)
transformation
done
clear
B)
genetic characters
done
clear
C)
embryonic induction
done
clear
D)
stimulation
done
clear
View Answer play_arrow
question_answer 227) Role of chromophill cells of Earthworm pharynx is:
A)
fat storage
done
clear
B)
helps in excretion
done
clear
C)
cocoon formation
done
clear
D)
saliva secretion
done
clear
View Answer play_arrow
question_answer 228) Which muscle pulls the leg in Rabbit?
A)
extensor muscle
done
clear
B)
abductor muscle
done
clear
C)
aductor muscle
done
clear
D)
flexor muscle
done
clear
View Answer play_arrow
question_answer 229) Bud develops in an adult Hydra:
A)
1-2 hrs.
done
clear
B)
3-4 hrs
done
clear
C)
12 days
done
clear
D)
2-4 days
done
clear
View Answer play_arrow
question_answer 230) Study the following cells of Hydra? (1) nerve cells (2) sensory cells (3) nematocyst (4) gastrodermal cells
A)
only 2
done
clear
B)
only 1
done
clear
C)
1 and 2
done
clear
D)
3 and 4
done
clear
View Answer play_arrow
question_answer 231) The area where brown fats are maximum in infant neck will have more :
A)
Golgi bodies
done
clear
B)
Mitochondria
done
clear
C)
Chromophill
done
clear
D)
Smooth E.R.
done
clear
View Answer play_arrow
question_answer 232) The average life span of Ascnris in host body is:
A)
1 month
done
clear
B)
6 to 12 weeks
done
clear
C)
6 to 12 months
done
clear
D)
4 to 6 months
done
clear
View Answer play_arrow
question_answer 233) What occurs in gastrulation ?
A)
Division of zygote
done
clear
B)
Formation of blastocoel
done
clear
C)
Epiboly and emboly takes place
done
clear
D)
Disappearance of archenteron
done
clear
View Answer play_arrow
question_answer 234) Which endocrine gland called..., secretes calcitonin hormone which regulates the \[C{{a}^{++}}\] in blood by:
A)
thyroid-reduction in \[C{{a}^{++}}\] concentration
done
clear
B)
thyroid-increase in \[C{{a}^{++}}\] concentration
done
clear
C)
parathyroid-increases in \[C{{a}^{++}}\]concentration
done
clear
D)
parathyroid-reduction in \[C{{a}^{++}}\] concentration
done
clear
View Answer play_arrow
question_answer 235) Which one is not a character of Prototheria ?
A)
Mammary glands devoid of teats
done
clear
B)
Presence of teeth in embryo
done
clear
C)
Testes in scrotum
done
clear
D)
Beak in adults
done
clear
View Answer play_arrow
question_answer 236) Which of the following is not produced from mesoderm?
A)
Blood
done
clear
B)
Adrenal medulla
done
clear
C)
Heart
done
clear
D)
Adrenal cortex
done
clear
View Answer play_arrow
question_answer 237) Secretion of ilium mucose which stimulate the pancrease and liver:
A)
enterogastrone and enterokinin
done
clear
B)
secretin and enterogastrone
done
clear
C)
cholecystokinin and enterokinin
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 238) Sperms get fertilization ability when they come in contact with vaginal fluid reactions is:
A)
acrosome reaction
done
clear
B)
cortical reaction
done
clear
C)
capacitation
done
clear
D)
fertilizing and antifertilizing reaction
done
clear
View Answer play_arrow
question_answer 239) Which statement is not true for Ascaris?
A)
The incidence of Ascaris is more in children
done
clear
B)
Ascaris does not produce toxic substance
done
clear
C)
Upto 5, 000 Ascaris can be recorded in intestine
done
clear
D)
Invenitics prove to be more harmful than the adults
done
clear
View Answer play_arrow
question_answer 240) Ascaris causes weakness because :
A)
parasites ingest protein component of the food
done
clear
B)
parasites products toxic substances hindering enzyme activity
done
clear
C)
carbohydrate digestion is affected due to toxic substances produced
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 241) Which of the following one is not a snake?
A)
Rattle snake
done
clear
B)
Rat snake
done
clear
C)
Glass snake
done
clear
D)
Blind snake
done
clear
View Answer play_arrow
question_answer 242) Lubb-dup sound in heart arises due to:
A)
opening of semilunar, bicuspid and tricuspid valves
done
clear
B)
closure of semilunar, bicuspid and tricuspid valves
done
clear
C)
opening of tricuspid and bicuspid valves
done
clear
D)
closure of bicuspid and tricuspid valves
done
clear
View Answer play_arrow
question_answer 243) Which action is not performed by HCL during digestion?
A)
Destruction of bacteria
done
clear
B)
Continuation of salivary amylase action
done
clear
C)
Protection from food population
done
clear
D)
Bone dissolvement
done
clear
View Answer play_arrow
question_answer 244) What is correct for mitochondria?
A)
They can replicate themselves
done
clear
B)
They can increase shape and size
done
clear
C)
Both a and b are correct
done
clear
D)
Both a and b are incorrect
done
clear
View Answer play_arrow
question_answer 245) Which substance is generally absent from aquous humor?
A)
Ascorbic acid
done
clear
B)
Hyaloronic acid
done
clear
C)
Glucose
done
clear
D)
\[{{O}_{2}}\] and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 246) Which of the following pair is in correct?
A)
Pheretima-South India and Srilanka
done
clear
B)
Lumbricus-Europe and North America
done
clear
C)
Dravida and Magascolex - South India
done
clear
D)
Eutyphaeus - South America
done
clear
View Answer play_arrow
question_answer 247) In Ascaris infective and harmful larval stages are:
A)
first fourth larval stage
done
clear
B)
third and fourth stage
done
clear
C)
first and third larval stage
done
clear
D)
second and third larval stage
done
clear
View Answer play_arrow
question_answer 248) In which form maximum \[C{{O}_{2}}\] is carried by blood:
A)
in the form of bicarbonates
done
clear
B)
free \[C{{O}_{2}}\]
done
clear
C)
carbamino haemoglobin
done
clear
D)
oxyhaemoglobin
done
clear
View Answer play_arrow
question_answer 249) The cause of black water fever is:
A)
Plasmodium vivnx
done
clear
B)
Plasmodium falciparum
done
clear
C)
Plasmodium ovale
done
clear
D)
Plasmodium malariae
done
clear
View Answer play_arrow
question_answer 250) Bilayer of lipid in plasma membrane reduces expulsion of \[{{K}^{+}}\] or \[M{{g}^{++}}\] because these are;
A)
important in cytoplasmic concentration
done
clear
B)
important for hormone action
done
clear
C)
because of strong attraction from inner ions
done
clear
D)
important to disturb balance in ECF
done
clear
View Answer play_arrow
question_answer 251) Which of the following pair shows correctly in which substance is completely oxidized and amount of energy released?
A)
Sucrose- 78 ATP, frutose 6- phosphate - 40 ATP, acetyl CoA -12 ATP
done
clear
B)
Sucrose - 72 ATP, fructose 39 ATP, acetyl Co A-10 ATP
done
clear
C)
Sucrose - 76 ATP, frutose 6 - phosphate 40 ATP, acetyl CoA -10 ATP
done
clear
D)
Sucrose - 76 ATP, fructose 6 - phosphate - 39 ATP, acetyl CoA - 10 ATP
done
clear
View Answer play_arrow
question_answer 252) In mammalian perilymph, following is more than endolymph:
A)
\[{{C}^{++}}\]
done
clear
B)
\[{{K}^{+}}\]
done
clear
C)
\[F{{e}^{++}}\]
done
clear
D)
\[N{{a}^{++}}\]
done
clear
View Answer play_arrow
question_answer 253) Which one is odd?
A)
Sea squirt
done
clear
B)
Sea cucumber
done
clear
C)
Sea urchin
done
clear
D)
Sea star
done
clear
View Answer play_arrow
question_answer 254) In both plant cell and animal cell flagella are similar in:
A)
both have membrane over it
done
clear
B)
both have microtubule 2 + 9 arrangement
done
clear
C)
both are flexible
done
clear
D)
microtubules are 9 + 2
done
clear
View Answer play_arrow
question_answer 255) Incubation period of Plasmodium falciparum is:
A)
10 days
done
clear
B)
12 days
done
clear
C)
16 days
done
clear
D)
28 days
done
clear
View Answer play_arrow
question_answer 256) Regeneration in Hiydra can occurs in the part with :
A)
gastrodermis
done
clear
B)
mesogloea
done
clear
C)
epidermis
done
clear
D)
1.6 mm or more
done
clear
View Answer play_arrow
question_answer 257) State bird of Rajasthan is:
A)
Passer domesticus
done
clear
B)
Columba livia
done
clear
C)
Cariotis migriseps
done
clear
D)
Pavo cristatus
done
clear
View Answer play_arrow
question_answer 258) One DNA helix have :
A)
10 nucleotides and \[3.4\,\overset{\text{o}}{\mathop{\text{A}}}\,\] in length
done
clear
B)
20 nucleotides and \[3.4\,\overset{\text{o}}{\mathop{\text{A}}}\,\] in length
done
clear
C)
20 nucleotides and \[34\,\overset{\text{o}}{\mathop{\text{A}}}\,\] in length
done
clear
D)
10 nucleotides and \[34\,\overset{\text{o}}{\mathop{\text{A}}}\,\] in length
done
clear
View Answer play_arrow
question_answer 259) Which statement is wrong for enzymes ?
A)
Specific in their reaction
done
clear
B)
Do not disturb equilibrium
done
clear
C)
All are of protein
done
clear
D)
They change the direction of reaction
done
clear
View Answer play_arrow
question_answer 260) Which pair is wrong?
A)
\[N{{a}^{+}}\]- nerve impulse
done
clear
B)
\[C{{o}^{++}}\]- for growth lungs and skin
done
clear
C)
\[C{{o}^{++}}\]-bones and teeth formation and muscle contraction
done
clear
D)
\[Z{{n}^{++}}\]-essential component of protoplasm
done
clear
View Answer play_arrow
question_answer 261) Copulation of Earthworm lasts for:
A)
5-6 hrs
done
clear
B)
15 min
done
clear
C)
1 hrs
done
clear
D)
2-3 hrs
done
clear
View Answer play_arrow
question_answer 262) Main characteristics of Cnidaria is:
A)
diploblastic, radial symmetry and cnidoblast cells
done
clear
B)
triploblastic, radial symmetry and cnidoblast cells
done
clear
C)
diploblastic, bilateral symmetry and cnidoblast cells
done
clear
D)
triploblastic, bilaterally symmetrical and cnidoblast cells
done
clear
View Answer play_arrow
question_answer 263) Metamerically segmented body means:
A)
body is divided externally on the basis of shape and colour
done
clear
B)
body is divided into head thorax, tail
done
clear
C)
repetation of segments
done
clear
D)
repetation of organ sequence in body
done
clear
View Answer play_arrow
question_answer 264) Which is the longest muscles which brings thigh near mid line?
A)
Chorocobranchiasis
done
clear
B)
Biceps femories
done
clear
C)
Castronimus
done
clear
D)
Sartorius
done
clear
View Answer play_arrow
question_answer 265) Where does ghost cells destroyed in Plasmodium infection in humans?
A)
Liver of man
done
clear
B)
Crop of mosquito
done
clear
C)
R.B.C. of man
done
clear
D)
Spleen of man
done
clear
View Answer play_arrow
question_answer 266) In 1994 Nobel prize for the discovery of protein in cell membrane was awarded to:
A)
Fischer and Krebs
done
clear
B)
Martin Loother and Nelson
done
clear
C)
Stuart and Bital
done
clear
D)
Gilman and Rodkil
done
clear
View Answer play_arrow
question_answer 267) Mesogloea of Hydra is chemically:
A)
mucoprotein
done
clear
B)
lipid
done
clear
C)
protein
done
clear
D)
mucopolysaccharide
done
clear
View Answer play_arrow
question_answer 268) What conditions are optimum for activation of gametogenesis in Plasmodiuni in stomach/alimentary canal of female Anopheles?
A)
High temperature
done
clear
B)
Presence of enzymes
done
clear
C)
Less temperature
done
clear
D)
Blood in stomach
done
clear
View Answer play_arrow
question_answer 269) Which one is main barrier in transportation in mitochondria that maintains composition of matrix?
A)
Inner membrane
done
clear
B)
Outer membrane
done
clear
C)
Perimitochondrial fluid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 270) Which one is not a correct pair?
A)
Cell-Schwann
done
clear
B)
Protoplasm-Huxley
done
clear
C)
Chromosome-Mendel
done
clear
D)
Germplasm-Weisman
done
clear
View Answer play_arrow
question_answer 271) Ambystoma larva of Tiger Salamander shows neoteny, because:
A)
fresh water becomes available due to rains
done
clear
B)
excess of mineral ions and \[{{l}_{2}}\]
done
clear
C)
less \[{{l}_{2}}\] and \[{{H}_{2}}O\]
done
clear
D)
hormone secretion starts from glands
done
clear
View Answer play_arrow
question_answer 272) If one incisors of Rabbit is replaced then:
A)
digestion will stop
done
clear
B)
rabbit will die after sometimes
done
clear
C)
digestion is troubled
done
clear
D)
nothing will happen
done
clear
View Answer play_arrow
question_answer 273) A structure absent in male reproductive system of Ascaris:
A)
testes
done
clear
B)
spermatic duct
done
clear
C)
seminal vesicle
done
clear
D)
ejaculatory duct
done
clear
View Answer play_arrow
question_answer 274) Largest invertebrate is:
A)
little fish
done
clear
B)
ship worm
done
clear
C)
devil fish
done
clear
D)
giant squid
done
clear
View Answer play_arrow
question_answer 275) Which of the following pair is wrong?
A)
Hypothalamus-thermoregulation
done
clear
B)
Medulla oblongata-control of in voluntay activities
done
clear
C)
Cerebellum-balance
done
clear
D)
Diencephalon-hunger and statiety center
done
clear
View Answer play_arrow
question_answer 276) State animal of Rajasthan is:
A)
Camelus
done
clear
B)
Ardeotis
done
clear
C)
Corvus
done
clear
D)
Gazella
done
clear
View Answer play_arrow
question_answer 277) Externally male and female Cockroach can be differentiated by:
A)
wingless condition
done
clear
B)
length of abdomen
done
clear
C)
length of maxillae
done
clear
D)
conglobate gland
done
clear
View Answer play_arrow
question_answer 278) Functionally heart of Cockroach is:
A)
neurogenic
done
clear
B)
agenic
done
clear
C)
epigenic
done
clear
D)
myogenic
done
clear
View Answer play_arrow
question_answer 279) In embryonic stage ammonia is converts to urea by:
A)
yolk sac
done
clear
B)
chorion
done
clear
C)
amnion
done
clear
D)
allantois
done
clear
View Answer play_arrow
question_answer 280) Assistance provided during locomotion in Amoeba:
A)
rough surface
done
clear
B)
nematocyst
done
clear
C)
tantaclcs
done
clear
D)
tons present in \[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 281) Those cells lipid is actively synthesized contains more:
A)
nucleus
done
clear
B)
R.E.R.
done
clear
C)
S.E.R.
done
clear
D)
golgi body
done
clear
View Answer play_arrow
question_answer 282) In 1902 Nobel prize was given to:
A)
Grassi for observing plasmodium in RBC
done
clear
B)
Laveron for discovering Plasmodium
done
clear
C)
Sir Ronald Ross for observing Plasmodium
done
clear
D)
Golgi for discovering eryhrocytic cycle
done
clear
View Answer play_arrow
question_answer 283) Biological clock is exhibited in Cockroach:
A)
in different stages of life cycle
done
clear
B)
in metamorphosis
done
clear
C)
as metabolic changes takes place in 2 hrs
done
clear
D)
in different activies in different season
done
clear
View Answer play_arrow
question_answer 284) For the parasympathetic nervous system (1) acetylcholine is the hormonal secretory product (2) fight and flight is controlled (3) pre-gangolic fibers are long and post gangolic are short (4) pre-gangolic fibers are short and post gangolic are long (5) origin from craniosacral region Find out the true statement:
A)
1, 2, 3, 5
done
clear
B)
1, 2, 4, 5
done
clear
C)
1, 4, 5
done
clear
D)
1, 3, 5
done
clear
View Answer play_arrow
question_answer 285) Malleus, in cus, stapes are modifications of respectively:
A)
articular, quadrate and Hyomandibular
done
clear
B)
quadrate, articular and hyomandibular
done
clear
C)
articular, hyomandibular and quadrate
done
clear
D)
hyomandibular, quadrate and articular
done
clear
View Answer play_arrow
question_answer 286) Which of the following group is hermaphrodite?
A)
Earthworm, Cockroach and Prawn
done
clear
B)
Earthworm, Leech, Taenia
done
clear
C)
Ascaris, Cockroach, Earthworm
done
clear
D)
Hydra, Ascaris. Taenia
done
clear
View Answer play_arrow
question_answer 287) Vit. D is synthesized in human being with the help of:
A)
ergosterol
done
clear
B)
ergocalciferol
done
clear
C)
cholesterol
done
clear
D)
carotene
done
clear
View Answer play_arrow
question_answer 288) Which of the following is true for internal fertilization?
A)
Presence of copulatory organs is essential
done
clear
B)
Water is essential (i.e. aquatic habitat is essential)
done
clear
C)
Semination occurs inside the female body
done
clear
D)
Male and female must not come close
done
clear
View Answer play_arrow
question_answer 289) Few scientist state vas deferens as seminal vesicle and seminal vesicle as vas deferens, because;
A)
sperms are not stored in seminal vesicle
done
clear
B)
their structure is similar
done
clear
C)
their function is same
done
clear
D)
sperms mature in vas deferens
done
clear
View Answer play_arrow
question_answer 290) Life span of Earthworm is:
A)
1-3 yrs
done
clear
B)
2-6 yrs
done
clear
C)
3 ½-10 yrs
done
clear
D)
61/6-15 yrs
done
clear
View Answer play_arrow
question_answer 291) Interstitial epithelium is protected from harmful bacteria by:
A)
goblet cells
done
clear
B)
brunners gland
done
clear
C)
peyers patches
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 292) VIIth cranial nerve enters in a ganglion called:
A)
gasserian ganglion
done
clear
B)
geniculate ganglion
done
clear
C)
vagus ganglion
done
clear
D)
cilliac ganglion
done
clear
View Answer play_arrow
question_answer 293) What does not happens when people listen to loud music?
A)
cochlear fluid oscillates at high degree
done
clear
B)
receptors of cochlear get degenerate
done
clear
C)
organ of corti react by the tympanic membrane
done
clear
D)
basal membrane vibrate to a large extent
done
clear
View Answer play_arrow
question_answer 294) In saliva of mosquito, following is present during its bite:
A)
xyote
done
clear
B)
gametocyte
done
clear
C)
ookinite
done
clear
D)
anticoagulant
done
clear
View Answer play_arrow
question_answer 295) When butter is kept in the air at room temperature then it start bad smelling because:
A)
it is saturated
done
clear
B)
it is unsaturated
done
clear
C)
bacterial action degrades it
done
clear
D)
it have large number of it \[{{H}_{2}}\] atoms
done
clear
View Answer play_arrow
question_answer 296) According to Heldane effect how oxyhaemoglobin behaves:
A)
It act as powerful base so reaction with \[C{{O}_{2}}\] reduces
done
clear
B)
It act as strong base so reaction with \[C{{O}_{2}}\]increase
done
clear
C)
Oxyhaemoglobin act as strong acid so association with \[C{{O}_{2}}\] reduces
done
clear
D)
Oxyhaemoglobin act as strong acid so association with \[C{{O}_{2}}\] increase
done
clear
View Answer play_arrow
question_answer 297) Adipose tissue in the skin is found in the:
A)
dermis
done
clear
B)
malpighian layer
done
clear
C)
epidermal layer
done
clear
D)
subcutaneous layer
done
clear
View Answer play_arrow
question_answer 298) Armadillo produces quadraplet because:
A)
sperms fertilizes four eggs
done
clear
B)
blastocyst divides into four cells
done
clear
C)
four sperms fertilizes ovum
done
clear
D)
four zygotes are formed
done
clear
View Answer play_arrow
question_answer 299) Which of the statement is correct for vitamin D?
A)
It is argosterol
done
clear
B)
It is important for teeth and bone development
done
clear
C)
Deficiency causes chilosis
done
clear
D)
It is a calciferol which controls \[C{{a}^{++}}\] and \[PO_{4}^{-}\] absorption from intestine
done
clear
View Answer play_arrow
question_answer 300) Which is the characteristic feature of ookinete?
A)
It have microtubules.
done
clear
B)
It have more mitochondria and ribosomes
done
clear
C)
It have contractile fibres
done
clear
D)
It is non-motile
done
clear
View Answer play_arrow
question_answer 301) Central dogma was proposed by:
A)
Mendel
done
clear
B)
Wastson and Crick
done
clear
C)
Crick
done
clear
D)
Schleiden and Schwann
done
clear
View Answer play_arrow
question_answer 302) Disease caused by fungus is:
A)
bronchitis
done
clear
B)
ringworm
done
clear
C)
small pox
done
clear
D)
tuberculosis
done
clear
View Answer play_arrow
question_answer 303) Y-chromosome present in:
A)
male
done
clear
B)
female
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 304) Enzymes for autolysis are found in:
A)
nucleus
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 305) In bacterial cell enzymes for aerobic respiration are found in:
A)
ribosome
done
clear
B)
mesosome
done
clear
C)
mitochundria
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 306) Phycology is the study of:
A)
fungus
done
clear
B)
algae
done
clear
C)
lichens
done
clear
D)
bryophyte
done
clear
View Answer play_arrow
question_answer 307) The common reaction in aerobic and anaerobic respiration is:
A)
fermentation
done
clear
B)
glycolysis
done
clear
C)
Krobs cycle
done
clear
D)
electron transport system
done
clear
View Answer play_arrow
question_answer 308) Connecting link of characters between one generation to another generation is :
A)
nucleus
done
clear
B)
nucleic acid
done
clear
C)
chromosome
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 309) Which type of cell division takes place during wound healing;
A)
Meiosis
done
clear
B)
Mitosis
done
clear
C)
Amitosis
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 310) CAM plants ecologically comes under which of the following category?
A)
Succulent plants
done
clear
B)
Mangrove plants
done
clear
C)
Halopliytes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 311) First stable substance of Calvin cycle is:
A)
phosphoglyceric acid
done
clear
B)
phosphoglyceraldehyde
done
clear
C)
oxato acetic acid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 312) Virus is classified in which of the following class?
A)
Akaryota
done
clear
B)
Prokaryota
done
clear
C)
Eukaryota
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 313) In nucleic acid what is responsible for genetic information?
A)
Quantity of nucleic acid
done
clear
B)
Type of nucleic acid
done
clear
C)
Linear sequence of nucleotide
done
clear
D)
Type of nucleotide
done
clear
View Answer play_arrow
question_answer 314) Membraneless cell organelle is:
A)
ribosome
done
clear
B)
lysosome
done
clear
C)
mitochondria
done
clear
D)
chloroplast
done
clear
View Answer play_arrow
question_answer 315) Female reproductive organ of Riccia is known as:
A)
Oedogonium
done
clear
B)
archegonium
done
clear
C)
carpogonium
done
clear
D)
oosphere
done
clear
View Answer play_arrow
question_answer 316) Casparian strip found in:
A)
cortex cells
done
clear
B)
pericycle
done
clear
C)
epidermis
done
clear
D)
endodermis
done
clear
View Answer play_arrow
question_answer 317) The study of organisms with their environment is called:
A)
Ecology
done
clear
B)
Cytology
done
clear
C)
Gene ecology
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 318) Botanical name of Chili is:
A)
Solanum melongena
done
clear
B)
Lycopercicum esculentum
done
clear
C)
Capsicum annuum
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 319) The reduction of \[C{{O}_{2}}\] occurs in:
A)
Calvin cycle
done
clear
B)
Krebs cycle
done
clear
C)
Glycolysis
done
clear
D)
Citric acid cycle
done
clear
View Answer play_arrow
question_answer 320) Plasmids present in:
A)
virus
done
clear
B)
mycoplasma
done
clear
C)
bacteria
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 321) In which of the following reserved food material is stored in the form of glycogen?
A)
Fungi
done
clear
B)
Algae
done
clear
C)
Virus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 322) Term ecosystem was proposed by :
A)
Tansley
done
clear
B)
Odum
done
clear
C)
HaeckeI
done
clear
D)
Mishra
done
clear
View Answer play_arrow
question_answer 323) The number of hydrogen bonds present between guanine and cytosine are :
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 324) Who proposed the histogen theory?
A)
Nagelli
done
clear
B)
Schmidt
done
clear
C)
Strassburger
done
clear
D)
Hanstein
done
clear
View Answer play_arrow
question_answer 325) The number of chloroplast in every cell of Ulothrix is:
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 326) On cutting of herbaceous stem of well irrigated plant, sap comes out because of the:
A)
turgor pressure
done
clear
B)
root pressure
done
clear
C)
osmotic pressure
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 327) The exception of Mendels law of dominance is:
A)
Pisum sativum
done
clear
B)
Mirabilis Jalapa
done
clear
C)
Cicer arietinum
done
clear
D)
Iberis amara
done
clear
View Answer play_arrow
question_answer 328) Cross between first filial generation \[({{F}_{1}})\] and pure recessive are called:
A)
test cross
done
clear
B)
back cross
done
clear
C)
dihybird cross
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 329) Which of the following nitrogenous base present only in RNA?
A)
Adenine
done
clear
B)
Uracil
done
clear
C)
Thymine
done
clear
D)
Guanine
done
clear
View Answer play_arrow
question_answer 330) Drug which reduce blood pressure is?
A)
Gum
done
clear
B)
Reserpine
done
clear
C)
Papaver
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 331) Curcuma longa belongs to family:
A)
Zingiberaceae
done
clear
B)
Apocynaceae
done
clear
C)
Umbelliferae
done
clear
D)
Papaveraceae
done
clear
View Answer play_arrow
question_answer 332) The source of opium is:
A)
stem
done
clear
B)
pinna
done
clear
C)
root
done
clear
D)
fruit
done
clear
View Answer play_arrow
question_answer 333) Annual rings are formed is:
A)
dicot herb
done
clear
B)
dicot stem
done
clear
C)
monocot stem
done
clear
D)
monocot root
done
clear
View Answer play_arrow
question_answer 334) Root nodules arc formed in family:
A)
Cruciferae
done
clear
B)
Leguminosae
done
clear
C)
Solanaceae
done
clear
D)
Poaceae
done
clear
View Answer play_arrow
question_answer 335) Salt from soil enters into plant cell in the form of:
A)
molecules
done
clear
B)
salts
done
clear
C)
atoms
done
clear
D)
ions
done
clear
View Answer play_arrow
question_answer 336) Energy source which does not emit \[C{{O}_{2}}\] in atmosphere is?
A)
Coal
done
clear
B)
Oil
done
clear
C)
Nuclear reactor
done
clear
D)
Natural gases
done
clear
View Answer play_arrow
question_answer 337) Anthocyanin pigment is found in:
A)
cell sap
done
clear
B)
chloroplast
done
clear
C)
mitochondria
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 338) Vacuoles are covered by:
A)
plasma membrane
done
clear
B)
tonoplast
done
clear
C)
Icucoplast
done
clear
D)
cell wall
done
clear
View Answer play_arrow
question_answer 339) Extra chromosomal DNA is found in:
A)
cytoplasm
done
clear
B)
mitochondria
done
clear
C)
golgi body
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 340) Glycolysis takes place in:
A)
mitochondria
done
clear
B)
cytoplasm
done
clear
C)
golgi body
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 341) The cell organelle covered by single unit membrane is:
A)
Lysosome
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 342) Water is not necessary for fertilization in which of the following?
A)
Pteridiim
done
clear
B)
Spirogyra
done
clear
C)
Marchantia
done
clear
D)
Albugo
done
clear
View Answer play_arrow
question_answer 343) Which of the following is not trace element?
A)
Mg
done
clear
B)
Zn
done
clear
C)
B
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 344) The largest herbarium of India is situated :
A)
In Dehradun
done
clear
B)
In Lucknow
done
clear
C)
In Calcutta
done
clear
D)
In Delhi
done
clear
View Answer play_arrow
question_answer 345) The flower of family Liliaceae is:
A)
hypogynous
done
clear
B)
synandrus
done
clear
C)
epigynous
done
clear
D)
perigynous
done
clear
View Answer play_arrow
question_answer 346) The connecting link between glycolysis and Krebs cycle is:
A)
acetyle Co-A
done
clear
B)
succinic acid
done
clear
C)
fumeric acid
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 347) The plant part which can respire without oxygen is?
A)
Root
done
clear
B)
Stem
done
clear
C)
Seed
done
clear
D)
Leaf
done
clear
View Answer play_arrow
question_answer 348) Archegonia is not present in:
A)
Riccia
done
clear
B)
Pteridium
done
clear
C)
Cycas
done
clear
D)
Capsella
done
clear
View Answer play_arrow
question_answer 349) In middle lamella, which substance is present in large quantity :
A)
cuitin
done
clear
B)
suberin
done
clear
C)
pectin
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 350) Crossing over takes place in:
A)
amitotic division
done
clear
B)
mitotic division
done
clear
C)
meiotic division
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 351) According to Mendels law of segregation the ratio of \[{{F}_{2}}\] generation is:
A)
1 : 1
done
clear
B)
3 : 1
done
clear
C)
5 : 2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 352) Rooted, submerged hydrophytic plant is:
A)
Eichhornia
done
clear
B)
Hydrilla
done
clear
C)
Lemna
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 353) Name the plant whose all cells are capable of gamete formation?
A)
Riccia
done
clear
B)
Marchantia
done
clear
C)
Albugo
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 354) Maximum energy is liberated by which of the following reaction?
A)
Glycolysis
done
clear
B)
Krebs cycle
done
clear
C)
Calvin cycle
done
clear
D)
Hatch and Slack cycle
done
clear
View Answer play_arrow
question_answer 355) Which of the following shows the undeveloped roots and lack of stomata?
A)
Halophytes
done
clear
B)
Xerophytes
done
clear
C)
Hydrophytes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 356) Vessels and companion cells are absent in:
A)
gymnosperm and pteridophyta
done
clear
B)
pteridophyta and monocot
done
clear
C)
dicot and angiosperms
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 357) Non vascular embryonic plant is:
A)
thallophyta
done
clear
B)
pteridophyta
done
clear
C)
bryophyta
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 358) The process of making the milk bacterialess is called:
A)
immunization
done
clear
B)
pasteurization
done
clear
C)
vernalization
done
clear
D)
purification
done
clear
View Answer play_arrow
question_answer 359) How many nuclei takes part in double fertilization?
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 360) The characteristic of Gossypium stamen is:
A)
monadelphous
done
clear
B)
biadelphous
done
clear
C)
polyadelphous
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 361) Which of the following is floral formula of mustard ?
A)
\[Ebr,\,\oplus \,\,_{\begin{smallmatrix} O \\ + \end{smallmatrix}}^{{}}\,{{K}_{2+2}}\,{{C}_{\times 4}}\,{{A}_{2+}}\,{{G}_{(\underline{2})}}\]
done
clear
B)
\[\,\oplus \,\,{{A}_{2}}\,{{C}_{3}}\,{{G}_{(\underline{2})}}\]
done
clear
C)
\[Ebr{{}_{O}}{{K}_{2+}}{{C}_{\times 3}}{{A}_{2+4}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 362) The fruit of Capsella is:
A)
caryopsis
done
clear
B)
silicula
done
clear
C)
lomantum
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 363) Who coined the term mitochondria?
A)
C. Benda
done
clear
B)
Altman
done
clear
C)
Kollciker
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 364) Endodermis is a part of:
A)
pericycle
done
clear
B)
cortex
done
clear
C)
epidermis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 365) Chromoplast is found in:
A)
stem
done
clear
B)
leaf
done
clear
C)
root
done
clear
D)
fruit and corolla of flower
done
clear
View Answer play_arrow
question_answer 366) Prosopis spicigera is a state tree of Rajasthan. Its family is:
A)
Mimosoideae
done
clear
B)
Caesalpinioideae
done
clear
C)
Papilionaceae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 367) Streptococcus is responsible for:
A)
conversion of alcohol from molasis
done
clear
B)
processing of leather
done
clear
C)
conversion of milk into curd
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 368) In an angiospermic plant, female gametophyte is represented by:
A)
ovule
done
clear
B)
embryo sac
done
clear
C)
endosperm
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 369) Which of the following is not renewable resource of India?
A)
fossils and mineral petrol
done
clear
B)
animals and minerals
done
clear
C)
plants and animals
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 370) How many male nuclei takes part in double fertilization of Capsella?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 371) In dorsal leaf, protoxylem is situated:
A)
in middle
done
clear
B)
towards upper epidermis
done
clear
C)
towards lower epidermis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 372) How many cells are present in mature male gametophyte of Capsella?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 373) Vivipary is present in:
A)
Peepal
done
clear
B)
Neem
done
clear
C)
Rhizophora
done
clear
D)
Jamoon
done
clear
View Answer play_arrow
question_answer 374) Axile placentation is present in:
A)
Cruciferae and Malvaceae
done
clear
B)
Malvaceae, Liliaceae and Solanaceae
done
clear
C)
Liliaceae and Cruciferae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 375) The causal agent of witches broom disease of Potato is:
A)
virus
done
clear
B)
mycoplasma
done
clear
C)
bacteria
done
clear
D)
fungus
done
clear
View Answer play_arrow
question_answer 376) Red drop is associated with pigment:
A)
chlorophyll-a-660
done
clear
B)
chlorophyll-a-680
done
clear
C)
chlorophyll-a-560
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 377) In Vallisneria stomata are present in:
A)
abaxial surface
done
clear
B)
adaxial surface
done
clear
C)
both dorsal and ventral surface
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 378) Conjoint collateral or bicollateral and open vascular bundle is the characteristic of:
A)
monocot stem
done
clear
B)
dicot stem
done
clear
C)
monocot root
done
clear
D)
dicot root
done
clear
View Answer play_arrow
question_answer 379) How many cells are present in female gametophyte of Capsella just before fertilization?
A)
2
done
clear
B)
3
done
clear
C)
5
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 380) Passage cells are present in:
A)
epidermis
done
clear
B)
cortex
done
clear
C)
endodermis
done
clear
D)
pericycle
done
clear
View Answer play_arrow
question_answer 381) The pigments of \[\text{PS-I}\] are:
A)
\[{{\text{P}}_{\text{700,}}}\] chlorophy\[\text{II-a,}\] carotenoids
done
clear
B)
\[{{P}_{680}}\]chlorophy\[\text{II-a,}\] xanthophyll
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 382) \[C{{O}_{2}}\] reduction results into:
A)
Co-A
done
clear
B)
pyruvic acid
done
clear
C)
citric acid
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 383) Which type of movement is present in Mimosa pudica?
A)
seismonastic
done
clear
B)
nyctinastic
done
clear
C)
chemonastic
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 384) The last structure of gametophytic stage in Riccia is:
A)
gamete
done
clear
B)
spore
done
clear
C)
spore mother cell
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 385) The unit of recombination is:
A)
muton
done
clear
B)
recon
done
clear
C)
cistron
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 386) In aquatic ecosystem which of the following is present in large number?
A)
producers
done
clear
B)
primary consumer
done
clear
C)
decomposers
done
clear
D)
secondary consumers
done
clear
View Answer play_arrow
question_answer 387) A genome contains:
A)
haploid chromosomal set
done
clear
B)
diploid chromosomal set
done
clear
C)
tetraploid chromosomal set
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 388) How much energy is liberated if 1 mole glucose is completly oxidized?
A)
6860 cal
done
clear
B)
68600 cal
done
clear
C)
686000 cal
done
clear
D)
6S60000 cal
done
clear
View Answer play_arrow
question_answer 389) When a cross made between pure red and pure white flowers of pea plant, than 120 plants are obtained the ratio of them is:
A)
30 red : 90 white
done
clear
B)
90 red : 30 white
done
clear
C)
60 red : 60 white
done
clear
D)
all red
done
clear
View Answer play_arrow
question_answer 390) Thigmotropism is shown by:
A)
tendril
done
clear
B)
pinna
done
clear
C)
stem
done
clear
D)
leaf
done
clear
View Answer play_arrow
question_answer 391) Flagella present in zoospores of Albugo are:
A)
two dissimilar, on lateral surface
done
clear
B)
two similar, on lateral surface
done
clear
C)
two similar, on anterior tip
done
clear
D)
two dissimilar, on anterior tip
done
clear
View Answer play_arrow
question_answer 392) Plants with higher osmotic pressure of cell sap are called :
A)
xerophyte
done
clear
B)
halophyte
done
clear
C)
hydrophyte
done
clear
D)
mesophyte
done
clear
View Answer play_arrow
question_answer 393) Which of the following have largest flower?
A)
Rafflesia
done
clear
B)
Water melon
done
clear
C)
Mango
done
clear
D)
Banana
done
clear
View Answer play_arrow
question_answer 394) The thorn of water chestnut (Trapa bipinosa) is the modification of:
A)
petals
done
clear
B)
sepals
done
clear
C)
stamens
done
clear
D)
style
done
clear
View Answer play_arrow
question_answer 395) The relation between Zoochlorella and Hydra is known as:
A)
endozoic
done
clear
B)
parasitism
done
clear
C)
heterotroph
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 396) Which of the following gas is responsible for add rain?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
None
done
clear
View Answer play_arrow
question_answer 397) The genotypic ratio of monohybrid generation will be:
A)
3 : 1
done
clear
B)
1: 2 : 1
done
clear
C)
1 : 1 : 1 : 1
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 398) The term cytokinin was coined by:
A)
Letham
done
clear
B)
Mendel
done
clear
C)
de Vries
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 399) Aflatoaxins are mainly produced by:
A)
algae
done
clear
B)
fungi
done
clear
C)
bryophyta
done
clear
D)
pteridophyta
done
clear
View Answer play_arrow
question_answer 400) The reserved food of Albugo is:
A)
glycogen
done
clear
B)
cellulose
done
clear
C)
starch
done
clear
D)
none of these
done
clear
View Answer play_arrow
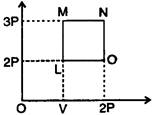
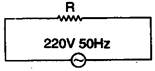
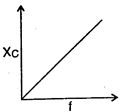
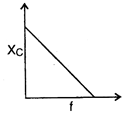
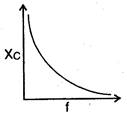
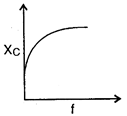
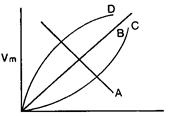
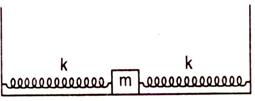
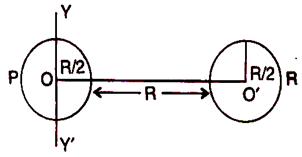
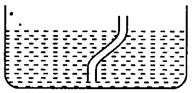 straight capillary when it is dipped vertically in water. If this capillary is now twisted as shown m figure. The height of water column will be:
straight capillary when it is dipped vertically in water. If this capillary is now twisted as shown m figure. The height of water column will be:
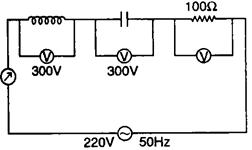
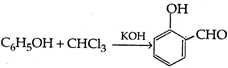 In the above reaction the intermediate species are:
In the above reaction the intermediate species are:
