question_answer 1) The power loss in pure inductor in an A.C. circuit will be:
A)
\[{{V}_{rms}}\]\[{{I}_{rms}}\]
done
clear
B)
more
done
clear
C)
zero
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 2)
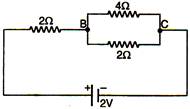
The potential difference across BC in the following figure will he:
A)
1.2 V
done
clear
B)
2 V
done
clear
C)
0.8 V
done
clear
D)
1 V
done
clear
View Answer play_arrow
question_answer 3) The distance between the ends of wings of an aeroplane is 5 m. The aeroplane is moving with velocity of 200 km/sec in a magnetic field of 10 T. The emf induced across the ends of wings will be:
A)
\[{{10}^{7}}\] volt
done
clear
B)
10 volt
done
clear
C)
\[{{10}^{6}}\] volt
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 4) Between the proton and neutron in a nucleus:
A)
nuclear force is greater than gravitational force
done
clear
B)
gravitational force is greater than nuclear force
done
clear
C)
electromagnetic force is lesser than gravitational force
done
clear
D)
nuclear force is greater than electromagnetic force
done
clear
View Answer play_arrow
question_answer 5) Potentiometer measures:
A)
potential difference
done
clear
B)
internal resistance
done
clear
C)
current
done
clear
D)
external resistance
done
clear
View Answer play_arrow
question_answer 6) The magnetic field \[\vec{B}\] in a circular coil does not depend on:
A)
current
done
clear
B)
radius
done
clear
C)
number of turns
done
clear
D)
area
done
clear
View Answer play_arrow
question_answer 7) Results obtained due to eddy currents is:
A)
high efficiency between coils of Trans former
done
clear
B)
low efficiency between coils of transformer
done
clear
C)
loss of heat in core of trans former
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 8) The radius of gyration of a hollow spherical shell is 2.5 J. If its frequency of rotation is made 10 times, then new kinetic energy will be:
A)
250 J
done
clear
B)
0.25 J
done
clear
C)
2500 J
done
clear
D)
2.5 J
done
clear
View Answer play_arrow
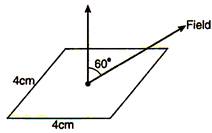
question_answer 9)
if a square coil is making an angle \[60{}^\circ \] with electric field E according to figure the electric flux passing through the square coil is (the side of square is 4 cm):
A)
853 E
done
clear
B)
8 E
done
clear
C)
16 E
done
clear
D)
none of these
done
clear
View Answer play_arrow
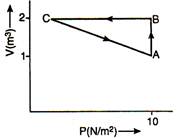
question_answer 10)
An ideal gas is carried along the cycle ABCA as shown in figure. The net heat given to gas in cycle is 5J. In process\[C\to A,\] the work done by the gas is:
A)
-5J
done
clear
B)
-10 J
done
clear
C)
-20 J
done
clear
D)
-15 J
done
clear
View Answer play_arrow
question_answer 11) The transition corresponding to first line of Balmer series will be:
A)
\[{{n}_{2}}\to {{n}_{1}}\]
done
clear
B)
\[{{n}_{3}}\to {{n}_{2}}\]
done
clear
C)
\[{{n}_{6}}\to {{n}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 12) The moment of inertia of a solid sphere about its tangential axis will be:
A)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
B)
\[\frac{7}{5}M{{R}^{2}}\]
done
clear
C)
\[\frac{5}{3}M{{R}^{2}}\]
done
clear
D)
\[\frac{2}{3}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 13) The time period of a vibrating spring due to a mass m is T. For twice of its mass, the new time period will be:
A)
\[\sqrt{2}T\]
done
clear
B)
\[2\sqrt{2}T\]
done
clear
C)
\[4T\]
done
clear
D)
\[5T\]
done
clear
View Answer play_arrow
question_answer 14) On a magnetic needle placed in a uniform magnetic field:
A)
\[F\ne 0,\,\,\,\tau \ne 0\]
done
clear
B)
\[F\ne 0,\,\,\,\tau =0\]
done
clear
C)
\[F=0,\,\,\,\tau \ne 0\]
done
clear
D)
\[F=0,\,\,\,\tau =0\]
done
clear
View Answer play_arrow
question_answer 15) The total energy of disc of mass M and velocity v rolling an inclined plane without slipping is:
A)
\[\frac{3}{4}M{{\upsilon }^{2}}\]
done
clear
B)
\[\frac{2}{5}M{{\upsilon }^{2}}\]
done
clear
C)
\[\frac{3}{2}M{{\upsilon }^{2}}\]
done
clear
D)
\[1\frac{1}{3}M{{\upsilon }^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) The path of a charged particle entering perpendicularly in a uniform electric field will be:
A)
linear
done
clear
B)
parabolic
done
clear
C)
circular
done
clear
D)
elliptical
done
clear
View Answer play_arrow
question_answer 17) The vector projection of a vector \[3\hat{i}+4\hat{k}\]on y-axis is:
A)
5
done
clear
B)
4
done
clear
C)
3
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 18) In an A.C. circuit containing capacitor:
A)
the current leads the potential difference in phase
done
clear
B)
the potential difference leads the current in phase
done
clear
C)
the potential difference and current are m phase
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) The work function of a metal is:
A)
the energy for the electron to enter into the metal
done
clear
B)
the energy for producing X-ray
done
clear
C)
the energy for the electron to come out from metal, surface
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 20) In nuclear fission, 0.1% mass is converted into energy. The energy released by the fission of 1 kg mass is:
A)
\[2.5\times {{10}^{7}}\,kWh\]
done
clear
B)
\[2.5\,\times {{10}^{3}}kWh\]
done
clear
C)
\[2.5\,\times {{10}^{6}}kWh\]
done
clear
D)
\[2\times {{10}^{8}}kWh\]
done
clear
View Answer play_arrow
question_answer 21) The pressure maintained in Coolidge tube is:
A)
\[{{10}^{-3}}\,mm\] mm of Hg
done
clear
B)
\[{{10}^{-7}}\,mm\] of Hg
done
clear
C)
10 mm of Hg
done
clear
D)
\[{{10}^{-4}}\,mm\] of Hg
done
clear
View Answer play_arrow
question_answer 22) The resistance of an ideal voltmeter is:
A)
very low
done
clear
B)
infinite
done
clear
C)
zero
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 23) The amplitude of a particle executing SHM is made three-fourth keeping its lime period constant. Its total energy will be:
A)
\[\frac{E}{2}\]
done
clear
B)
\[\frac{3}{4}E\]
done
clear
C)
\[\frac{9}{16}E\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
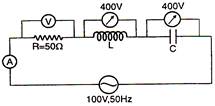
question_answer 24)
In the series LCR circuit, the voltmeter and ammeter readings are:
A)
2 V, 100 A
done
clear
B)
100 V, 2A
done
clear
C)
100 V, 100 A
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 25) The average power loss in a resistor in A.C. circuit is:
A)
zero
done
clear
B)
\[{{P}_{rms}}\]\[{{I}_{rms}}\]
done
clear
C)
infinite
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 26) If in an R-L circuit, \[{{X}_{L}}=100\Omega \] and \[R=100\,\Omega ,\] then in this circuit:
A)
current leads the potential difference in phase by \[90{}^\circ \]
done
clear
B)
current lags behind the potential difference in phase by \[45{}^\circ \]
done
clear
C)
current leads the potential difference in phase by \[45{}^\circ \]
done
clear
D)
current and potential difference are in same phase
done
clear
View Answer play_arrow
question_answer 27) In a p-type semiconductor, there are mainly:
A)
free electrons
done
clear
B)
holes
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 28) On increasing the temperature of semiconductor, its resistance :
A)
decreases
done
clear
B)
increases
done
clear
C)
depends on pressure
done
clear
D)
does not depend on temperature
done
clear
View Answer play_arrow
question_answer 29) In a triode valve, grid is used to control the plate current, because:
A)
grid has negative voltage
done
clear
B)
grid has no voltage
done
clear
C)
grid is nearer to cathode than plate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 30) When an electron in an hydrogen atom makes a transition from first Bohr orbit to second Bohr orbit, how much energy it absorbs?
A)
3.4 eV
done
clear
B)
10.2 eV
done
clear
C)
13.6 eV
done
clear
D)
1.51 eV
done
clear
View Answer play_arrow
question_answer 31) If in a diode valve, the plate potential becomes double, then plate current will be:
A)
\[2\sqrt{2}\] times
done
clear
B)
8 times
done
clear
C)
\[3\sqrt{3}\] times
done
clear
D)
2 times
done
clear
View Answer play_arrow
question_answer 32) If temperature becomes double, the emitted radiation will be :
A)
16 times
done
clear
B)
8 times
done
clear
C)
\[2\sqrt{2}\] times
done
clear
D)
32 times
done
clear
View Answer play_arrow
question_answer 33) The kinetic energy of one mole gas at 300 K temperature, is E. At 400 K temperature kinetic energy is \[E.\] The value of \[E/E\] is:
A)
1.33
done
clear
B)
\[\sqrt{\left( \frac{4}{3} \right)}\]
done
clear
C)
\[\frac{16}{9}\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 34) The moment of inertia in rotational motion will be equivalent to ....... as in linear motion:
A)
mass
done
clear
B)
velocity
done
clear
C)
momentum
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 35) If temperature becomes triple, the root mean square velocity of gas molecules will be:
A)
\[\upsilon /2\]
done
clear
B)
\[\upsilon \sqrt{3}\]
done
clear
C)
\[\sqrt{3}\upsilon \]
done
clear
D)
same \[[\upsilon \] is the root mean square velocity of gas molecules at temperature T]
done
clear
View Answer play_arrow
question_answer 36)
If a sphere rolling on an inclined plane with velocity v without slipping, the vertical height of the incline in terms of velocity will be:
A)
\[\frac{7\upsilon }{10g}\]
done
clear
B)
\[\frac{7{{\upsilon }^{2}}}{10g}\]
done
clear
C)
\[\frac{2{{\upsilon }^{2}}}{5g}\]
done
clear
D)
\[\frac{2\upsilon }{5g}\]
done
clear
View Answer play_arrow
question_answer 37) Two substances have different atomic masses and same atomic number. They are:
A)
isotopes
done
clear
B)
isobars
done
clear
C)
isotones
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 38) The value of susceptibility of a diamagnetic substance is:
A)
small positive
done
clear
B)
small negative
done
clear
C)
positive
done
clear
D)
negligible
done
clear
View Answer play_arrow
question_answer 39) In isothermal process, the work done in expansion of gas from volume \[{{V}_{1}}\] to \[{{V}_{2}}\] will be:
A)
\[nRT\,\ln \left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\]
done
clear
B)
\[nRT\,\left( \ln \frac{{{V}_{1}}}{{{V}_{2}}} \right)\]
done
clear
C)
\[nRT\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 40) A proton moving with velocity\[\upsilon \]and an \[\text{-}\]particle experience the same force in a magnetic field. The velocity of the \[\alpha \]-particle is:
A)
\[\frac{\upsilon }{8}\]
done
clear
B)
\[4\upsilon \]
done
clear
C)
\[\upsilon \]
done
clear
D)
\[\frac{\upsilon }{2}\]
done
clear
View Answer play_arrow
question_answer 41) A spaceship of weight 39200 kg-W lands on planet mars whose radius is \[3.4\times {{10}^{6}}m\] and mass is \[6.42\,\times {{10}^{23}}kg\]. The weight of space ship on mars will be (surface tension of water \[=7\times {{10}^{-2}}N/m\]):
A)
5000 kg-W
done
clear
B)
10000 kg-W
done
clear
C)
15000 kg-W
done
clear
D)
20000 kg-W
done
clear
View Answer play_arrow
question_answer 42) The diameter of one drop of water is 0.2 cm. The work done in breaking one drop into 100 droplets will be:
A)
\[7.9\times {{10}^{-6}}J\]
done
clear
B)
\[5.92\times {{10}^{-6}}J\]
done
clear
C)
\[2.92\times {{10}^{-6}}J\]
done
clear
D)
\[1.92\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 43) A geostationary satellite is rotating in circular orbit of radius 36000 km around the earth. A spy satellite which is rotating in circular orbit at a height of some hundred kilometre from earths surface, has time period approximately equal to (Re = 6400 km):
A)
1 hour
done
clear
B)
2 hour
done
clear
C)
24 hour
done
clear
D)
36 hour
done
clear
View Answer play_arrow
question_answer 44) The degrees of freedom of a stationary rigid body about its axis will be:
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 45) The velocity of simple pendulum is maximum at:
A)
extremes
done
clear
B)
half displacement
done
clear
C)
mean position
done
clear
D)
every where
done
clear
View Answer play_arrow
question_answer 46) The separation between the two charges \[+q\]and\[-q\] becomes double. The value of force will be:
A)
two fold
done
clear
B)
half
done
clear
C)
four fold
done
clear
D)
one fourth
done
clear
View Answer play_arrow
question_answer 47) A body is executing SHM. Which of the following properties remains same at each point of its path during motion?
A)
Acceleration
done
clear
B)
Velocity
done
clear
C)
Phase
done
clear
D)
Total energy
done
clear
View Answer play_arrow
question_answer 48) If two coils have self inductance \[{{L}_{1}}\] and\[{{L}_{2}}\] the coefficient of mutual induction will be:
A)
\[M\propto \sqrt{{{L}_{1}}{{L}_{2}}}\]
done
clear
B)
\[M\propto \sqrt{\left( \frac{{{L}_{1}}}{{{L}_{2}}} \right)}\]
done
clear
C)
\[M\propto \sqrt{\left( \frac{{{L}_{2}}}{{{L}_{1}}} \right)}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 49) When a glass rod is rubbed with silk, the amount of positive charge acquired by glass rod in magnitude is:
A)
less than the charge on silk
done
clear
B)
greater than the charge on silk
done
clear
C)
equal to me charge on silk
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 50) Two sound sources \[{{S}_{1}}\] and\[{{S}_{2}}\]of frequencies 324 Hz and 320 Hz are placed at certain separation. An observer is moving away from\[{{S}_{1}}\] and towards \[{{S}_{2}}\] on line joining them. If he hears no beats then speed of observer is (\[\upsilon \]= 344m/sec):
A)
20 m/s
done
clear
B)
10 m/s
done
clear
C)
5 m/s
done
clear
D)
2.1 m/s
done
clear
View Answer play_arrow
question_answer 51) Which of the following statements is not true about reactance of capacitor?
A)
It is positive
done
clear
B)
It is proportional to capacitance
done
clear
C)
It is inversely proportional to capacitance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 52) The current in pure inductor in an A.C. circuit:
A)
leads the potential difference in phase by \[90{}^\circ \]
done
clear
B)
lags behind the potential difference in phase by \[90{}^\circ \]
done
clear
C)
leads the potential difference in phase by \[45{}^\circ \]
done
clear
D)
lags behind the potential difference in phase by \[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 53) The hot wire ammeter measures:
A)
D.C. current
done
clear
B)
A.C. current
done
clear
C)
none of above
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 54) In a step-up transformer ratio of number of turns in primary to seconday coil is 1 : 25. If input voltage is 230 volt and load current is 2 amp, then current in primary is :
A)
50 A
done
clear
B)
25 A
done
clear
C)
12.5 A
done
clear
D)
6.25 A
done
clear
View Answer play_arrow
question_answer 55) Thermal speed of free electrons is of the order of :
A)
\[{{10}^{-5\,}}\,m/s\]
done
clear
B)
\[{{10}^{2}}\,m/s\]
done
clear
C)
\[{{10}^{8}}\,m/s\]
done
clear
D)
\[{{10}^{5}}\,m/s\]
done
clear
View Answer play_arrow
question_answer 56) At the time of total solar eclipse, the spectrum of solar radiation will have :
A)
All Fraunhofer lines changed into bright coloured lines
done
clear
B)
no lines at all
done
clear
C)
a smaller number of dark Fraunhofer lines
done
clear
D)
a large number of dark Fraunhofer lines
done
clear
View Answer play_arrow
question_answer 57) The displacement equation of a particle is \[x=3\,\sin \,2t+4\,\cos \,2t,\] where \[x\] is in metre and t in second. The amplitude and maximum velocity will be respectively:
A)
5m, 10 m/s
done
clear
B)
3m, 2 m/s
done
clear
C)
4m, 2 m/s
done
clear
D)
3m, 4 m/s
done
clear
View Answer play_arrow
question_answer 58) The radius of first Bohr orbit is \[0.5\,\overset{\text{o}}{\mathop{\text{A}}}\,,\] then radius of fourth Bohr orbit will be:
A)
\[0.03\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.12\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2.0\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[8.0\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 59) In order that a thin film of oil floating on die surface of water shows colours due to interference, the thickness of the oil film should be of the order of:
A)
\[10000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[50000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[10\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1\, \ cm\]
done
clear
View Answer play_arrow
question_answer 60) The temperature of an ideal gas at atmospheric pressure is 300K and volume1m3.If temperature and volume become double, then pressure will be:
A)
\[{{10}^{5}}N/{{m}^{2}}\]
done
clear
B)
\[2\times {{10}^{5}}N/{{m}^{2}}\]
done
clear
C)
\[0.5\times {{10}^{5}}N/{{m}^{2}}\]
done
clear
D)
\[4\times {{10}^{5}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 61)
The system of three charges is shown in figure. If charge B is displaced slightly towards A, then:
A)
it will displace more towards A
done
clear
B)
it will displace towards C
done
clear
C)
its path will be parabolic
done
clear
D)
its motion will be SHM
done
clear
View Answer play_arrow
question_answer 62) It will displace more towards A it will displace towards C its path will be parabolic its motion will be SHM The lengths of two closed organ pipes are 0.750 m and 0.770 m. If they are sounded together, 3 beats per second are produced. The velocity of sound will be:
A)
330.5 m/sec
done
clear
B)
340.5 m/sec
done
clear
C)
346.5 m/sec
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 63) A traveller is running towards a stationary train. If the train sounds a horn of frequency n, the apparent frequency heard by the traveller will be:
A)
\[n=n\left( \frac{\upsilon +{{\upsilon }_{0}}}{\upsilon } \right)\]
done
clear
B)
\[n=n\left( \frac{\upsilon -{{\upsilon }_{0}}}{\upsilon +{{\upsilon }_{s}}} \right)\]
done
clear
C)
\[n=n\left( \frac{\upsilon +{{\upsilon }_{0}}}{\upsilon } \right)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 64) The susceptibility of ferromagnetic and paramagnetic substances are respectively:
A)
positive, positive
done
clear
B)
positive, negative
done
clear
C)
negative, positive
done
clear
D)
negative, negative
done
clear
View Answer play_arrow
question_answer 65) On increasing the temperature of a gas contained in a closed vessel by \[1{}^\circ C\], the pressure increases by 0.4%. The initial temperature of gas is:
A)
25 K
done
clear
B)
250 K
done
clear
C)
\[2500{}^\circ C\]
done
clear
D)
\[250{}^\circ C\]
done
clear
View Answer play_arrow
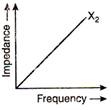
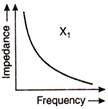
question_answer 66)
The graphs given below depict the dependence of two reactive impedances\[{{X}_{1}}\]and\[{{X}_{2}}\] on the frequency of the alternating emf applied individually to them. We can then say that:
A)
\[{{X}_{1}}\] is an inductor and \[{{X}_{2}}\] is a capacitor
done
clear
B)
\[{{X}_{1}}\] is a resistor and \[{{X}_{2}}\] is a capacitor
done
clear
C)
\[{{X}_{1}}\] is a capacitor and \[{{X}_{2}}\] is an inductor
done
clear
D)
\[{{X}_{1}}\] is an inductor and \[{{X}_{2}}\] is a resistor
done
clear
View Answer play_arrow
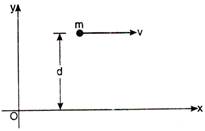
question_answer 67)
A mass m is moving with constant velocity along a line parallel to \[x-\]axis beyond origin. Its angular momentum about die origin:
A)
remains constant
done
clear
B)
is zero
done
clear
C)
increases
done
clear
D)
decreases
done
clear
View Answer play_arrow
question_answer 68) A particle executes SUM with frequency The frequency of its kinetic energy is:
A)
\[f\]
done
clear
B)
\[2f\]
done
clear
C)
\[f/2\]
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 69) Three spheres have radii 7.5 cm, 8.5 cm and 9.5 cm respectively. Which sphere exerts maximum pressure on earth?
A)
First
done
clear
B)
Second
done
clear
C)
Third
done
clear
D)
All same
done
clear
View Answer play_arrow
question_answer 70) In an ideal transformer:
A)
\[{{P}_{output}}<{{P}_{input}}\]
done
clear
B)
\[{{P}_{output}}>{{P}_{input}}\]
done
clear
C)
\[{{P}_{output}}><{{P}_{input}}\]
done
clear
D)
\[{{P}_{output}}={{P}_{input}}\]
done
clear
View Answer play_arrow
question_answer 71) What will be the angular width of central maximum in Fraunhofer diffraction when light of wavelength \[6000\overset{\text{o}}{\mathop{\text{A}}}\,\] is used and slit width is \[12\times {{10}^{-5}}cm\]?
A)
2 rad
done
clear
B)
3 rad
done
clear
C)
1 rad
done
clear
D)
8 rad
done
clear
View Answer play_arrow
question_answer 72) A spring executes SHM with mass of 10 kg attached to it. The force constant of spring is 10Nm. If at any instant its velocity is 40 cm/sec, the displacement will be (where amplitude is 0.5m):
A)
0.09 m
done
clear
B)
0.3 m
done
clear
C)
0.03 m
done
clear
D)
0.9 m
done
clear
View Answer play_arrow
question_answer 73) A train moving with 20 m/s towards a stationary observer produces frequency of 440 Hz. The apparent frequency heard will be (\[\upsilon \] = 330 m/s):
A)
448 Hz
done
clear
B)
455 Hz
done
clear
C)
440 Hz
done
clear
D)
468 Hz
done
clear
View Answer play_arrow
question_answer 74) A thin prism P of angle \[4{}^\circ \] made of glass of refractive index 1.54 is combined to a thin prism Q made of glass of refractive index 1.72 to produce dispersion without deviation. The angle of prism Q is:
A)
\[4{}^\circ \]
done
clear
B)
\[3{}^\circ \]
done
clear
C)
\[2.6{}^\circ \]
done
clear
D)
\[5.3{}^\circ \]
done
clear
View Answer play_arrow
question_answer 75) The de-Broglie wavelength\[\lambda \]:
A)
is proportional to mass
done
clear
B)
is proportional to impulse
done
clear
C)
inversely proportional to impulse
done
clear
D)
does not depend on impulse
done
clear
View Answer play_arrow
question_answer 76) In an LCR (parallel) A.C. circuit, the impedance in state of resonance is:
A)
equal to resistance
done
clear
B)
zero
done
clear
C)
infinite
done
clear
D)
depending on L and C
done
clear
View Answer play_arrow
question_answer 77) The average power lost in pure capacitor in an A.C. circuit:
A)
is zero
done
clear
B)
depends on V
done
clear
C)
depends on C
done
clear
D)
depends on insulator placed in parallel plate capacitor
done
clear
View Answer play_arrow
question_answer 78) 78. The plate resistance of triode valve is \[30k\Omega \] and trans conductance is 2x10-3 mho. The amplification factor is:
A)
60
done
clear
B)
50
done
clear
C)
1.5
done
clear
D)
0.67
done
clear
View Answer play_arrow
question_answer 79) For making p-n junction diode forward biased:
A)
same potential is applied
done
clear
B)
greater potential is given to n compared to p
done
clear
C)
greater potential is given to p compared to n
done
clear
D)
unbalanced concentration
done
clear
View Answer play_arrow
question_answer 80) The half life of radioactive substance is 4 days. Its 100 g is kept for 16 days. After this period, the amount of substance remained is:
A)
25g
done
clear
B)
15 g
done
clear
C)
10 g
done
clear
D)
6.25g
done
clear
View Answer play_arrow
question_answer 81) Hysteresis property is shown by:
A)
ferromagnetic substances
done
clear
B)
paramagnetic substances
done
clear
C)
both above
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 82) A nucleus of mass number 232 and atomic number 90 after many disintegrations of \[\alpha \] and \[\] radiations, decays into other nucleus whose mass number is 220 and atomic number is 86. The numbers of \[\alpha \] and \[\beta \] radiations will be:
A)
4, 0
done
clear
B)
3, 6
done
clear
C)
3, 2
done
clear
D)
2, 1
done
clear
View Answer play_arrow
question_answer 83) If at temperature \[{{T}_{1}}=1000K,\] the wavelength is \[1.4\times {{10}^{-6}}m,\] then at what temperature the wavelength will be \[2.8\times {{10}^{-6}}m\]?
A)
2000 K
done
clear
B)
500 K
done
clear
C)
250 K
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) The minimum wavelength of photon is \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]its energy will be:
A)
2.5 eV
done
clear
B)
50 V
done
clear
C)
5.48 eV
done
clear
D)
7.48 eV
done
clear
View Answer play_arrow
question_answer 85) The potential of dipole at its axial position is proportional to distance r as:
A)
\[{{r}^{-2}}\]
done
clear
B)
\[{{r}^{-1}}\]
done
clear
C)
r
done
clear
D)
\[{{r}^{o}}\]
done
clear
View Answer play_arrow
question_answer 86) The \[\beta \]-particles are emitted by:
A)
atom
done
clear
B)
orbit
done
clear
C)
nucleus
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 87) If in a Youngs double slit experiment, the slit width is 3 cm, the separation between slits and screen is 7 cm and wavelength of light is \[1000\overset{\text{o}}{\mathop{\text{A}}}\,,\]then fringe width will be (\[\alpha =1{}^\circ \],\[\mu \] = 1.5):
A)
\[2\times {{10}^{-5\,}}\,m\]
done
clear
B)
\[2\times {{10}^{-3\,}}\,m\]
done
clear
C)
\[0.2\times {{10}^{-5\,}}\,m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 88) The direction of Lorentz force can be found by:
A)
Flemings right hand rule
done
clear
B)
Flemings left hand rule
done
clear
C)
Maxwells screw law
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 89) The dielectric constant K of an insulator can be:
A)
5
done
clear
B)
0.5
done
clear
C)
-1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 90) Four equal charges q are placed at centre of a conducting hollow sphere. If they are displaced 1.5 cm from centre, the change in flux will be:
A)
doubled
done
clear
B)
tripled
done
clear
C)
same
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 91) How is the time period of simple pendulum vary with its amplitude?
A)
\[T\propto a\]
done
clear
B)
\[T\propto {{a}^{2}}\]
done
clear
C)
Does not depend on its amplitude
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 92) If length of wire is 2m and the mass is 80 kg, the tension in wire will be if frequency is \[\frac{1}{4}\]Hz:
A)
400 N
done
clear
B)
80 N
done
clear
C)
4 N
done
clear
D)
4000 N
done
clear
View Answer play_arrow
question_answer 93) The value of heat produced by the water if \[\mu =100,\] \[{{C}_{p}}=1.5\] and temperature difference is \[20{}^\circ C\], is:
A)
3000 J
done
clear
B)
300 J
done
clear
C)
7.5 J
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 94) When charge is given to a soap bubble, it shows:
A)
an increase in size
done
clear
B)
sometimes an increase and sometimes a decrease in size
done
clear
C)
no change in size
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 95) When temperature of a gas is increased then which of the following statements is always true?
A)
Work is done on the gas
done
clear
B)
Heat is supplied to gas
done
clear
C)
Energy of gas is increased
done
clear
D)
Pressure of gas remains unchanged.
done
clear
View Answer play_arrow
question_answer 96) An increase in pressure required to decrease the 200 litres volume of a liquid by 0.004% in pipa is (Bulk modulus of the liquid = 2100 Mpa)
A)
188 kPa
done
clear
B)
8.4 kPa
done
clear
C)
18.8 kPa
done
clear
D)
84 kPa
done
clear
View Answer play_arrow
question_answer 97) The number of bullets are fired in all possible directions with the same initial velocity n. The maximum area of ground covered by bullets is:
A)
\[\pi {{\left( \frac{2{{\mu }^{2}}}{g} \right)}^{2}}\]
done
clear
B)
\[2\pi {{\left( \frac{u}{g} \right)}^{2}}\]
done
clear
C)
\[\pi {{\left( \frac{u}{2g} \right)}^{2}}\]
done
clear
D)
\[\pi {{\left( \frac{{{u}^{2}}}{g} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 98) If on heating liquid through \[80{}^\circ C\],the mass expelled is (1/100) the of mass still remaining, the coefficient of apparent expansion of liquid is:
A)
\[1.25\times {{10}^{-4}}/{}^\circ C\]
done
clear
B)
\[12.5\times {{10}^{-4}}/{}^\circ C\]
done
clear
C)
\[152\times {{10}^{-5}}/{}^\circ C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 99) If 2g of helium is enclosed in a vessel at NTP, how much heat should be added to it to double the pressure?
A)
1638 J
done
clear
B)
1019 J
done
clear
C)
1568 J
done
clear
D)
836 J
done
clear
View Answer play_arrow
question_answer 100) Angular velocity of minute hand of a clock is:
A)
\[\frac{\pi }{30\,}\,rad/s\]
done
clear
B)
\[\pi \,rad/s\]
done
clear
C)
\[2\pi \,rad/s\]
done
clear
D)
\[\frac{\pi }{1800}\,rad/s\]
done
clear
View Answer play_arrow
question_answer 101) The correct IUPAC name of the following compound is: \[{{C}_{2}}{{H}_{5}}.\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,.{{C}_{2}}{{H}_{5}}\]
A)
3, 4-dimethyul-3-hyexne
done
clear
B)
3, 5-dimethyul-3-hyexne
done
clear
C)
3, 4-dimethyul-2-hyexne
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 102) Which of the following compound yield\[2,5-dimethyl\text{ }hexan-1,\text{ }6-dial\]?
A)
3, 6-dimethyl cyclohexene
done
clear
B)
3, 4-dimethyl cyclohexene
done
clear
C)
4, 6-dimethyl cyclohexene
done
clear
D)
3, 5-dimethyl cyclohexene
done
clear
View Answer play_arrow
question_answer 103) Which of these are isomers?
A)
Dimethyl ether and ethanol
done
clear
B)
Acetic acid and acetone
done
clear
C)
Acetic acid and acetaldehyde
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) Compound X \[({{C}_{3}}{{H}_{8}}O)\] on oxidation gives compound Y \[({{C}_{3}}{{H}_{6}}O)\]. What is compound X?
A)
\[{{1}^{o}}\text{ }alcohol\]
done
clear
B)
\[{{2}^{o}}\,alcohol\]
done
clear
C)
\[{{3}^{o}}\text{ }alcohol~\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 105) Which of the following easily accept proton?
A)
\[{{F}^{-}}\]
done
clear
B)
\[B{{r}^{-}}\]
done
clear
C)
\[O{{H}^{-}}\]
done
clear
D)
\[N{{H}_{2}}^{-}\]
done
clear
View Answer play_arrow
question_answer 106) The bond angle in \[R-OR\]is:
A)
\[{{110}^{o}}\]
done
clear
B)
\[{{109}^{o}}\]
done
clear
C)
\[108{}^\circ \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 107) \[C-O\] bond in ether can be broken by:
A)
\[HI\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
\[HF\]
done
clear
View Answer play_arrow
question_answer 108) Strongest \[C-H\] bond is present in:
A)
ethane
done
clear
B)
ethane
done
clear
C)
ethyne
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 109) In which of the following reactions, ethyl chloride is obtained in good yield?
A)
\[Ethane+C{{l}_{2}}\xrightarrow{h\omega }\]
done
clear
B)
\[Ethane+C{{l}_{2}}(excess)\xrightarrow{h\omega }\]
done
clear
C)
\[Ethane+C{{l}_{2}}\xrightarrow{in\,dark}\]
done
clear
D)
\[Benzene+C{{l}_{2}}\xrightarrow{{}}\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following yield 2 mole of formaldehyde on ozonolysis?
A)
\[CH\equiv CH\]
done
clear
B)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 111) Acetylene on treating with \[C{{u}_{2}}C{{l}_{2}}\]gives:
A)
\[C{{u}_{2}}{{C}_{2}}\]
done
clear
B)
\[CuCHCl\]
done
clear
C)
\[~C{{H}_{4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 112) What will be obtained when acetylene is treated with arsenic chloride?
A)
Lewisite
done
clear
B)
Mustard gas
done
clear
C)
Westron
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) Phenol on treatment with formaldehyde in dilute alkaline solution, gives:
A)
bakelite
done
clear
B)
terylene
done
clear
C)
nylon
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 114) Bradys reagent is:
A)
\[2,4-DNP\]
done
clear
B)
\[cone.\text{ }HCl+ZnC{{l}_{2}}\]
done
clear
C)
\[DMSO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 115) Hinsbergs reagent is used to distinguish between:
A)
\[{{1}^{o}}\] and \[{{2}^{o}}\] amines
done
clear
B)
\[{{2}^{o}}\] and \[{{3}^{o}}\] amines
done
clear
C)
(a) & (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 116) The reaction of \[{{H}_{2}}\] on ethene in presence of catalyst is called :
A)
Sabatier Sanderens reaction
done
clear
B)
Darzen reaction
done
clear
C)
Rosenmund reduction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 117) Propene on addition with \[HI,\] gives:
A)
\[C{{H}_{3}}-CHI-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}I\]
done
clear
C)
\[C{{H}_{3}}-CHI-C{{H}_{2}}I\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 118) Which of the following reacts with both acetaldehyde and acetone?
A)
F.S
done
clear
B)
\[C{{H}_{3}}MgBr\]
done
clear
C)
T.R
done
clear
D)
Benedict solution
done
clear
View Answer play_arrow
question_answer 119) Schiffs solution B is obtained when:
A)
sulphurous acid is passed through magenta dye
done
clear
B)
chlorine is passed through magenta dye
done
clear
C)
both (a) & (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 120) Fentons reagent is:
A)
\[FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[HgS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
done
clear
C)
\[FeS{{O}_{4}}+{{H}_{2}}O\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 121) Compound used in Rosenmunds reduction is:
A)
\[RCOCl\]
done
clear
B)
\[RCON{{H}_{2}}\]
done
clear
C)
\[RCOOR\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 122) Which of the following shows chloroform reaction?
A)
\[C{{H}_{3}}CHOHC{{H}_{3}}\]
done
clear
B)
Methanol
done
clear
C)
n-butanol
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 123) Which on reduction does not give primary amines?
A)
\[{{C}_{2}}{{H}_{3}}NC\]
done
clear
B)
\[C{{H}_{3}}CN\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 124) Primary amine \[+\text{ }aldehyde\xrightarrow{{}}X,\]X is:
A)
amino
done
clear
B)
imino
done
clear
C)
nitro
done
clear
D)
nitrito
done
clear
View Answer play_arrow
question_answer 125) The reactivity order of acid derivatives is:
A)
\[RCOCl>RCOOCOR>RCON{{H}_{2}}\]
done
clear
B)
\[RCOCl<RCOOCOR<RCON{{H}_{2}}\]
done
clear
C)
\[RCOCl>RCOOCOR<RCON{{H}_{2}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 126) \[Aniline+NaN{{O}_{2}}\text{+ }HCl\xrightarrow{{}}X,\]X is:
A)
diazo salt
done
clear
B)
dye
done
clear
C)
polymer
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 127) Which of the following reacts fastly with \[Na\]?
A)
\[{{1}^{o}}\text{ }alcohol\]
done
clear
B)
\[{{2}^{o}}\text{ }alcohol\]
done
clear
C)
\[{{3}^{o}}\text{ }alcohol\]
done
clear
D)
The reactivity of all is equal
done
clear
View Answer play_arrow
question_answer 128) Which of the following is the strongest base?
A)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 129) \[Phenol+CC{{l}_{4}}+KOH\xrightarrow{{}}\text{ }X,\]X is:
A)
salicyclic acid
done
clear
B)
salicaldehyde
done
clear
C)
benzoic acid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 130) Toluene \[\xrightarrow{Cr{{O}_{2}}C{{l}_{2}}}A\xrightarrow{anhydride}B,\]B is:
A)
cinnamic acid
done
clear
B)
cinnamaldehyde
done
clear
C)
salicyclic acid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 131) \[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}COC1\xrightarrow{AlC{{l}_{3}}}P,\]the product P is:
A)
acetophenone
done
clear
B)
benzophenone
done
clear
C)
toluene
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 132) \[C{{H}_{3}}C{{H}_{2}}COOH\xrightarrow{SOC{{l}_{2}}}A\] \[\xrightarrow{LiAl{{H}_{4}}\,(ter.\,but.\,oxide)}B\xrightarrow{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{|}}}\,}C.\] C is:
A)
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COCl\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 133) Lucas reagent is:
A)
\[Conc.HCl+ZnC{{l}_{2}}\]
done
clear
B)
\[N{{H}_{2}}\text{ }N{{H}_{2}}/{{C}_{2}}{{H}_{5}}ONa\]
done
clear
C)
\[Zn-Hg/HCl\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 134) Which of the following can distinguish between carbonyl compounds and carboxylic acids?
A)
\[NaHC{{O}_{3}}\]
done
clear
B)
\[NaHS{{O}_{4}}\]
done
clear
C)
\[Cone.\text{ }HCl\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 135) The last traces of water from alcohol can be removed by:
A)
passing dry \[HCl\]
done
clear
B)
ether
done
clear
C)
\[Mg\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 136) Aniline and benzene can be distinguished by:
A)
\[HCl\]
done
clear
B)
\[NaOH\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 137) Ethyl alcohol cannot be purified more than 95% by distillation, because:
A)
it forms H-bonds with water
done
clear
B)
it is lighter than water
done
clear
C)
it forms azeotropic solution
done
clear
D)
all the above are correct
done
clear
View Answer play_arrow
question_answer 138) Which of the following shows cis-trans isomerism?
A)
\[2-butyne\]
done
clear
B)
\[1-butyne\]
done
clear
C)
\[2-butene\]
done
clear
D)
\[1-butene\]
done
clear
View Answer play_arrow
question_answer 139) Why ethyl iodide becomes coloured when it is exposed to air?
A)
Iodine flows out
done
clear
B)
Iodine is liberated
done
clear
C)
lodoform is formed
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 140) The compound, which decolorize bromine water but does not react with Tollens reagent is:
A)
ethene
done
clear
B)
ethyne
done
clear
C)
propane
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 141) The compound which reacts with hydroxyl amine but does not react with Tollens reagent is:
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 142) Marsh gas is:
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{3}}{{H}_{8}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 143) Natural gas is:
A)
\[C{{H}_{4}}\]
done
clear
B)
\[CO+{{H}_{2}}+C{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}+{{C}_{2}}{{H}_{6}}+{{C}_{3}}{{H}_{8}}\]
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 144) \[CHC{{l}_{3}}\]is tested prior to its use as anaesthetic, by:
A)
\[AgN{{O}_{3}}+HCl\]
done
clear
B)
\[HCl\]
done
clear
C)
\[AgN{{O}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 145) Which is used to get ethyl alcohol from acetic acid?
A)
Acetobacter acetii
done
clear
B)
Zymase
done
clear
C)
Maltase
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 146) Which of the following does not give carbylamine test?
A)
\[\phi -N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
D)
all die above give this test
done
clear
View Answer play_arrow
question_answer 147) Westrosol is:
A)
trichloro ethylene
done
clear
B)
tetrachloro ethane
done
clear
C)
tetrachloro ethylene
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 148) Possible isomers of \[{{C}_{3}}{{H}_{7}}Cl\] are:
A)
\[2\]
done
clear
B)
\[5\]
done
clear
C)
\[3\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 149) \[CH\equiv CH+HgS{{O}_{4}}\xrightarrow{{{H}_{2}}O}A,\] A is:
A)
\[C{{H}_{2}}=C{{H}_{2}}~\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[HCHO\]
done
clear
D)
acetaldehyde
done
clear
View Answer play_arrow
question_answer 150) Bohrs model is:
A)
successful in calculating energy of helium like atoms
done
clear
B)
successful in calculating energy of hydrogen like atoms
done
clear
C)
successful in calculating energy of lithium like atoms
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 151) The energy of zero point in terms of principal quantum numbers is:
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 152) Which of the following statements is not true?
A)
The most appropriate level for electron in zero level is nucleus
done
clear
B)
The wave function of electron in an atom is related to its spinning speed
done
clear
C)
The electron does not have magnetic moment while spinning
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 153) Electronegativity is maximum of:
A)
\[N\]
done
clear
B)
\[Cl\]
done
clear
C)
\[C\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 154) Which of the following has maximum charge density?
A)
\[S{{c}^{2-}}\]
done
clear
B)
\[{{S}^{2-}}\]
done
clear
C)
\[{{O}^{2-}}\]
done
clear
D)
\[T{{e}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 155) Lewis acid is:
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[GaC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 156) pH of \[N/100\text{ }\] \[HCl\]will be:
A)
\[1\]
done
clear
B)
\[4\]
done
clear
C)
\[0.2\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 157) In a buffer, if the ratio of salt and acid is increased 10 times, then pH will:
A)
increase by 10
done
clear
B)
decrease by 10
done
clear
C)
increase by one
done
clear
D)
decrease by one
done
clear
View Answer play_arrow
question_answer 158) The solution of \[N{{a}_{2}}C{{O}_{3}}\] will be:
A)
weakly basic
done
clear
B)
strongly basic
done
clear
C)
weakly acidic
done
clear
D)
strongly acidic
done
clear
View Answer play_arrow
question_answer 159) The correct statement for isotonic solution is:
A)
both have equal pH on taking equal moles
done
clear
B)
both have equal \[pKa\]on taking equal moles
done
clear
C)
both have equal molar solubility
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 160) \[{{N}_{2}}+{{O}_{2}}2NO.\]. For this reaction \[{{K}_{a}}=100,\] then \[{{K}_{a}}\] for reaction, \[2NO{{N}_{2}}+{{O}_{2}}\]will be:
A)
\[0.01\]
done
clear
B)
\[0.1\]
done
clear
C)
\[10\]
done
clear
D)
\[100\]
done
clear
View Answer play_arrow
question_answer 161) Which reaction will proceed in forward direction on increasing pressure?
A)
\[C(s)+{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}(s)+0.5{{O}_{2}}\xrightarrow{{}}S{{O}_{3}}\]
done
clear
C)
\[{{N}_{2}}+3{{H}_{2}}\xrightarrow{{}}2N{{H}_{3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 162) The nature of the solution of potash alum is:
A)
acidic
done
clear
B)
basic
done
clear
C)
neutral
done
clear
D)
amphoteric
done
clear
View Answer play_arrow
question_answer 163) Lattice energy is calculated by :
A)
Born-Haber cycle
done
clear
B)
Mullikens equation
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 164) Which element is not found in nature?
A)
\[Tc\]
done
clear
B)
\[Y\]
done
clear
C)
\[Sr\]
done
clear
D)
\[Pt\]
done
clear
View Answer play_arrow
question_answer 165) In which of the following methods, \[Mg\] is used to reduce titanium chloride?
A)
IMI process
done
clear
B)
Haber process
done
clear
C)
Krolls method
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 166) In which of the following, there are 3 bp and 2 Ip?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[Cl{{F}_{3}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 167) Which is the coordinating solvent in the following reaction? \[B{{F}_{3}}+HF+{{H}_{2}}O\xrightarrow{{}}{{H}_{3}}{{O}^{+}}+BF_{4}^{-}\]
A)
\[HF\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 168) Which change is involved in the extraction of metal from metal oxide?
A)
Free energy change and pressure
done
clear
B)
Free energy change and temperature
done
clear
C)
Free energy change and concentration
done
clear
D)
Free energy change and volume
done
clear
View Answer play_arrow
question_answer 169) Maximum paramagnetism is in:
A)
\[C{{r}^{3+}}\]
done
clear
B)
\[M{{n}^{2+}}\]
done
clear
C)
\[M{{n}^{3+}}\]
done
clear
D)
\[N{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 170) Which of the following is wrong electronic configuration?
A)
\[Sc=3{{d}^{1}}{{s}^{2}}\]
done
clear
B)
\[Ti=3{{d}^{2}}{{s}^{2}}\]
done
clear
C)
\[Cr=3{{d}^{5}}{{s}^{1}}\]
done
clear
D)
\[V=3{{s}^{2}}{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 171) Which of the following give red-violet colour in flame-test?
A)
\[Li\]
done
clear
B)
\[Na\]
done
clear
C)
\[Cs\]
done
clear
D)
\[Rb\]
done
clear
View Answer play_arrow
question_answer 172) The reason for the light yellow colour of \[AgBr\]is:
A)
\[AgBr\] is more covalent due to more polarizing power of \[A{{g}^{+}}\]
done
clear
B)
\[AgBr\]is less covalent
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 173) Which of the following reaction is balanced?
A)
\[AsO_{4}^{2-}+MnO_{4}^{-}\xrightarrow{{}}AsO_{4}^{3-}+Mn{{O}_{2}}+2{{H}_{2}}O\]
done
clear
B)
\[MnO_{4}^{-}+{{C}_{2}}O_{4}^{2-}\xrightarrow{{}}M{{n}^{2+}}+C{{O}_{2}}\]
done
clear
C)
\[Cu+4HN{{O}_{3}}\xrightarrow{{}}Cu{{(N{{O}_{3}})}_{2}}+2N{{O}_{2}}+2{{H}_{2}}O\]
done
clear
D)
\[{{H}_{2}}S+HN{{O}_{3}}\to {{H}_{2}}O+NO+S\]
done
clear
View Answer play_arrow
question_answer 174) Which of the following oxide is acidic?
A)
\[{{B}_{2}}{{O}_{3}}\]
done
clear
B)
\[Be{{O}_{2}}\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 175) In the electrolysis of alumina, cryolite and \[Ca{{F}_{2}}\]are added to:
A)
increase the emf of cell
done
clear
B)
decrease the emf of cell
done
clear
C)
decrease the melting point
done
clear
D)
both (b) and (c)
done
clear
View Answer play_arrow
question_answer 176) Maximum unpaired electrons are in:
A)
\[C{{r}^{2+}}\]
done
clear
B)
\[M{{n}^{3+}}\]
done
clear
C)
\[M{{n}^{2+}}\]
done
clear
D)
\[C{{o}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 177) The ionization energy will be maximum for:
A)
\[H\]
done
clear
B)
\[Li\]
done
clear
C)
\[Be\]
done
clear
D)
\[B\]
done
clear
View Answer play_arrow
question_answer 178) Which oxidation state is the most common among lanthanides?
A)
\[+2\]
done
clear
B)
\[+3\]
done
clear
C)
\[+1\]
done
clear
D)
\[+4\]
done
clear
View Answer play_arrow
question_answer 179) The correct quantum numbers of 3p-electrons, are:
A)
\[n=3,l=2,m=+2,s=+\frac{1}{2}\]
done
clear
B)
\[n=3,l=1,m=-1,s=-\frac{1}{2}\]
done
clear
C)
\[n=3,l=-2,m=-2,s=+\frac{1}{2}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 180) The equilibrium constant \[{{K}_{C}}\] is affected by:
A)
temperature
done
clear
B)
pressure
done
clear
C)
initial concentration of reactants
done
clear
D)
catalyst
done
clear
View Answer play_arrow
question_answer 181) Which of the following reaction is an acid-base reaction?
A)
\[Ca{{C}_{2}}{{O}_{4}}+{{H}^{+}}\to C{{a}^{2+}}+H{{C}_{2}}O_{4}^{-}\]
done
clear
B)
\[Al+{{H}_{2}}O+O{{H}^{-}}\to Al{{O}_{2}}+\frac{3}{4}{{H}_{2}}\]
done
clear
C)
\[HgS+NO_{3}^{-}+{{H}^{+}}\to H{{g}^{2+}}+{{H}_{2}}O+....\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 182) Which of the following reaction is disproportionation?
A)
\[2{{H}_{2}}S+S{{O}_{2}}\to 2{{H}_{2}}O+3S\]
done
clear
B)
\[Ca+{{H}_{2}}\to Ca{{H}_{2}}\]
done
clear
C)
\[4P+3NaOH+3{{H}_{2}}O\to P{{H}_{3}}\]\[+3Na{{H}_{2}}P{{O}_{2}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 183) How many \[\sigma \] and \[\pi \]-bonds are in \[SO_{4}^{2-}\]?
A)
\[4,2\]
done
clear
B)
\[3,2\]
done
clear
C)
\[4,3\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 184) Which of the following is square planar?
A)
\[Cl{{F}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[{{[Pt{{(Cl)}_{4}}]}^{2-}}\]
done
clear
D)
\[HgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 185) The wrong match is:
A)
\[P{{F}_{3}}-\]trigonal bipyramidal
done
clear
B)
\[S{{O}_{2}}-\]angular
done
clear
C)
\[Cl{{F}_{3}}-\]tetrahedral
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 186) In a basic buffer \[0.0025\text{ }mole\]of \[N{{H}_{4}}Cl\] and \[0.15\text{ }mole\]of \[N{{H}_{4}}OH\]are present. The pH of the solution will be: \[{{(}_{P}}{{K}_{b}}=4.74)\]
A)
\[11.04\]
done
clear
B)
\[10.24\]
done
clear
C)
\[6.62\]
done
clear
D)
\[5.48\]
done
clear
View Answer play_arrow
question_answer 187) Whose electron affinity will be less than zero?
A)
\[{{O}^{2-}}\]
done
clear
B)
\[{{S}^{2-}}\]
done
clear
C)
both (a) and (b)
done
clear
D)
\[{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 188) Which of the following transition is correct for Balmer series?
A)
\[3\xrightarrow{{}}1\]
done
clear
B)
\[1\xrightarrow{{}}2\]
done
clear
C)
\[4\xrightarrow{{}}2\]
done
clear
D)
\[2\xrightarrow{{}}4\]
done
clear
View Answer play_arrow
question_answer 189) \[{{K}_{C}}=9\] for the reaction,\[A+BC+D\]. If A and B are taken in equal amounts, then amount of C in equilibrium is:
A)
\[1\]
done
clear
B)
\[0.25\]
done
clear
C)
\[0.75\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 190) Nylon is not a:
A)
condensation polymer
done
clear
B)
poly amide
done
clear
C)
copolymer
done
clear
D)
homopolymer
done
clear
View Answer play_arrow
question_answer 191) When amonia is heated with dry alkali metal in presence of \[Fe,\] the product obtained is:
A)
\[Fe,\] complex, \[{{H}_{2}}\]
done
clear
B)
metal amide, \[{{H}_{2}}\]
done
clear
C)
alkaline complex, \[{{H}_{2}}\]
done
clear
D)
alkaline complex
done
clear
View Answer play_arrow
question_answer 192) \[Mn{{O}_{2}}\xrightarrow{{}}M{{n}^{3+}}\] The oxidation state of \[Mn\] changes from:
A)
\[+4\xrightarrow{{}}+3\]
done
clear
B)
\[+4\xrightarrow{{}}+2\]
done
clear
C)
\[+2\xrightarrow{{}}+1\]
done
clear
D)
\[+3\xrightarrow{{}}+1\]
done
clear
View Answer play_arrow
question_answer 193) \[_{p}{{K}_{a}}\] will be maximum for:
A)
\[HCl{{O}_{4}}\]
done
clear
B)
\[HCl{{O}_{3}}\]
done
clear
C)
\[HCl{{O}_{2}}\]
done
clear
D)
\[HClO\]
done
clear
View Answer play_arrow
question_answer 194) The energy of first shell in hydrogen is \[13.6\text{ }eV,\]the energy of second shell will be:
A)
\[3.4eV\]
done
clear
B)
\[10.2\text{ }eV\]
done
clear
C)
\[1\,eV\]
done
clear
D)
\[3.02\text{ }eV\]
done
clear
View Answer play_arrow
question_answer 195) The method used to prepare steel is:
A)
Bessemers converter method
done
clear
B)
Seimens Martin process
done
clear
C)
Von-Arkel method
done
clear
D)
L.D. process
done
clear
View Answer play_arrow
question_answer 196) The number of \[\alpha -\] and \[\beta -\]particles lost when \[_{92}{{U}^{238}}\] changes to \[_{82}P{{b}^{206}}\] :
A)
\[8\alpha ,\,6\beta \]
done
clear
B)
\[6\alpha ,\,6\beta \]
done
clear
C)
\[6\alpha ,\,6\beta \]
done
clear
D)
\[4\alpha ,\,4\beta \]
done
clear
View Answer play_arrow
question_answer 197) Zeises salt is:
A)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
B)
\[K[PtC{{l}_{3}}\,({{C}_{2}}{{H}_{4}})]\]
done
clear
C)
\[Fe{{({{C}_{5}}{{H}_{5}})}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 198) How many Faradays are required to reduce one mole of \[MnO_{4}^{-}\] to \[M{{n}^{2+}}\]?
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 199) If a process is both endothennic and spontaneous, then:
A)
\[\Delta S>0\]
done
clear
B)
\[\Delta S<0\]
done
clear
C)
\[\Delta G>0\]
done
clear
D)
\[\Delta E=0\]
done
clear
View Answer play_arrow
question_answer 200)
Number of chiral carbon atoms in
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[1\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 201) Golgi complex works for:
A)
excretion
done
clear
B)
respiration
done
clear
C)
secretion
done
clear
D)
reduction
done
clear
View Answer play_arrow
question_answer 202) The number of chromosomes becomes half in:
A)
anaphase\[\text{-I}\]
done
clear
B)
anaphase\[\text{-II}\]
done
clear
C)
telophase\[\text{-I}\]
done
clear
D)
telophase\[\text{-II}\]
done
clear
View Answer play_arrow
question_answer 203) Lipid bilayer is present in:
A)
plasma membrane
done
clear
B)
ribosome
done
clear
C)
chromosome
done
clear
D)
nucleolus
done
clear
View Answer play_arrow
question_answer 204) Hydrolases are found in:
A)
lysosomes
done
clear
B)
ribosomes
done
clear
C)
mesosomes
done
clear
D)
peroxisomes
done
clear
View Answer play_arrow
question_answer 205) Chiasmata are formed:
A)
due to crossing over of some part between homologous chromosomes
done
clear
B)
due to crossing over of some part between non homologous chromosomes
done
clear
C)
due to duplication of homologous and non-homologous chromosomes
done
clear
D)
due to loss of some part of chromosomes
done
clear
View Answer play_arrow
question_answer 206) Acrosome is formed by:
A)
mitochondria
done
clear
B)
golgi body
done
clear
C)
ribosomes
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 207) In protoplasm fat store in the form of :
A)
polypeptide
done
clear
B)
triglyceride
done
clear
C)
polysaccharide
done
clear
D)
nucleosides
done
clear
View Answer play_arrow
question_answer 208) Antibody is formed by :
A)
protein
done
clear
B)
carbohydrate
done
clear
C)
nucleic acid
done
clear
D)
lipid
done
clear
View Answer play_arrow
question_answer 209) Food store as oil in:
A)
Chlamydomonas
done
clear
B)
Oedogonium
done
clear
C)
Vaucheria
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 210) BOD increased by:
A)
algae
done
clear
B)
moss
done
clear
C)
ferns
done
clear
D)
distilated wastes
done
clear
View Answer play_arrow
question_answer 211) Lowest number of chromosomes are found in:
A)
Haplopappus
done
clear
B)
Cyprus
done
clear
C)
Salix
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 212) Peat moss is :
A)
Funeria
done
clear
B)
ferns
done
clear
C)
algae
done
clear
D)
Sphagnum
done
clear
View Answer play_arrow
question_answer 213) Difference between algae and bryophyte is:
A)
terrestrial habitat
done
clear
B)
sterile jacket
done
clear
C)
biflagellate gametes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 214) Which have great importance in genetics?
A)
Penicillium
done
clear
B)
Clewceps
done
clear
C)
Neurospora
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 215) The protein toxin producing bacteria, which used to control biological pest is :
A)
E. coli
done
clear
B)
Agrobacterium
done
clear
C)
Mycobacterium sp.
done
clear
D)
B. thuringiensis
done
clear
View Answer play_arrow
question_answer 216) Bacteria with single flagella at one end is called :
A)
monotrichous
done
clear
B)
lophotrichous
done
clear
C)
amphitrichous
done
clear
D)
peritrichous
done
clear
View Answer play_arrow
question_answer 217) Bacteria causing disease of citrus is:
A)
citrus leaf spot
done
clear
B)
stunt disease
done
clear
C)
citrus canker
done
clear
D)
citrus greening leaf disease
done
clear
View Answer play_arrow
question_answer 218) \[{{O}_{2}}\] does not evolved in photosynthesis of:
A)
BGA
done
clear
B)
green algae
done
clear
C)
bacteria
done
clear
D)
autotrophic plant
done
clear
View Answer play_arrow
question_answer 219) Photosynthetic pigment of bacteria is:
A)
chromoplast
done
clear
B)
chloroplast
done
clear
C)
leucoplast
done
clear
D)
chromatophore
done
clear
View Answer play_arrow
question_answer 220) Virus have :
A)
RNA
done
clear
B)
DNA
done
clear
C)
Either RNA or DNA
done
clear
D)
Neither DNA nor RNA
done
clear
View Answer play_arrow
question_answer 221) Which group is evolutionary modern?
A)
Gymnosperms
done
clear
B)
Grasses
done
clear
C)
Pteridophytes
done
clear
D)
Algae
done
clear
View Answer play_arrow
question_answer 222) Absence of which is cause of naked seeds?
A)
Integument
done
clear
B)
Nucellus
done
clear
C)
Ovary wall
done
clear
D)
Perianth
done
clear
View Answer play_arrow
question_answer 223) Genes for haemophilia disease is located on:
A)
autosomes
done
clear
B)
mitochondria
done
clear
C)
chloroplasts
done
clear
D)
sex chromosomes
done
clear
View Answer play_arrow
question_answer 224) Most stable ecosystem is:
A)
desert
done
clear
B)
marine
done
clear
C)
mountain
done
clear
D)
forest
done
clear
View Answer play_arrow
question_answer 225) Stratification occurs in:
A)
desert
done
clear
B)
tropical forest
done
clear
C)
deciduous forest
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 226) Ecological pyramids were discovered by?
A)
Eiton
done
clear
B)
Odum
done
clear
C)
Reiter
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 227) Haptonastic movement is found in:
A)
Drosera
done
clear
B)
Oxalis
done
clear
C)
Mimosa
done
clear
D)
Cucurbita
done
clear
View Answer play_arrow
question_answer 228) Which one of the following was rediscoverer of Mendels work:
A)
Muller
done
clear
B)
Morgan
done
clear
C)
Correns
done
clear
D)
Bridge
done
clear
View Answer play_arrow
question_answer 229) In yeast during anaerobic respiration how many glucose molecules are required for production of 38 ATP?
A)
1
done
clear
B)
2
done
clear
C)
19
done
clear
D)
38
done
clear
View Answer play_arrow
question_answer 230) Krebs cycle is completed by:
A)
hydrogenation
done
clear
B)
oxidation
done
clear
C)
nitrification
done
clear
D)
reduction
done
clear
View Answer play_arrow
question_answer 231) Tulip belong to family:
A)
Asteraceae
done
clear
B)
Liliaceae
done
clear
C)
Brassicaceae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 232) Tetradynamous stamens are found in:
A)
Cruciferae
done
clear
B)
Amaryllidaceae
done
clear
C)
Mimoseae
done
clear
D)
Compositae
done
clear
View Answer play_arrow
question_answer 233) Bicarpellary, syncarpus and with pseudoseptum fruit is:
A)
siliqua
done
clear
B)
akene
done
clear
C)
capsule
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 234) Heroin is:
A)
diacetyl morphine
done
clear
B)
triacetyl morphine
done
clear
C)
tetra acetyl morphine
done
clear
D)
mono acetyl morphine
done
clear
View Answer play_arrow
question_answer 235) Conditions helpful in photo respiration are:
A)
more \[{{O}_{2}}\] and less \[C{{O}_{2}}\]
done
clear
B)
less \[{{O}_{2}}\] and more \[C{{O}_{2}}\]
done
clear
C)
more temperature and less \[{{O}_{2}}\]
done
clear
D)
more humidity and less temperature
done
clear
View Answer play_arrow
question_answer 236) Bicollateral bundles are found in:
A)
Cucurbitaceae
done
clear
B)
Malvaceae
done
clear
C)
Cruciferae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 237) Vascular bundle with cambium is called:
A)
closed
done
clear
B)
open
done
clear
C)
exarch
done
clear
D)
endarch
done
clear
View Answer play_arrow
question_answer 238) When phloem is surrounded completely by xylem the vascular bundle is called:
A)
amphicribral
done
clear
B)
amphivasal
done
clear
C)
conjoint
done
clear
D)
collateral
done
clear
View Answer play_arrow
question_answer 239) Lignification is associated with:
A)
xylem
done
clear
B)
phloem
done
clear
C)
parenchyma
done
clear
D)
chlorenchyma
done
clear
View Answer play_arrow
question_answer 240) Periderm included:
A)
Phellum, Phelloderm, Plerome
done
clear
B)
Phellum, Phellogen, Dermatogen
done
clear
C)
Phellum, Phellogen, Phelloderm
done
clear
D)
Phellum, Phellogen, Cortex
done
clear
View Answer play_arrow
question_answer 241) Which of the following is the result of double fertilization?
A)
Cotyledon
done
clear
B)
Nucellus
done
clear
C)
Endosperm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 242) Embryo sac is:
A)
megagametophyte
done
clear
B)
megasporophyte
done
clear
C)
megasporophyll
done
clear
D)
endosperm
done
clear
View Answer play_arrow
question_answer 243) Pollen tube entered through micropyle in :
A)
porogamy
done
clear
B)
chalazogamy
done
clear
C)
syngamy
done
clear
D)
misogamy
done
clear
View Answer play_arrow
question_answer 244) Which formed in oxidative phosphorylation?
A)
ATP in photosynthesis
done
clear
B)
ATP in respiration
done
clear
C)
\[NADP{{H}_{2}}\] in photosynthesis
done
clear
D)
\[NADP{{H}_{2}}\] in respiration
done
clear
View Answer play_arrow
question_answer 245) Which of the following is the region of cell division?
A)
Root cap
done
clear
B)
meristematic region
done
clear
C)
Root hair zone
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 246) Natural auxin is :
A)
NAA
done
clear
B)
\[\text{IAA}\]
done
clear
C)
2, 4-D
done
clear
D)
Aviton
done
clear
View Answer play_arrow
question_answer 247) How many letters used in genetic code?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 248) Correct name is :
A)
Brassica indica
done
clear
B)
Mengifera Indica
done
clear
C)
SOLANUM MELONGINA
done
clear
D)
Mimosa Pudica
done
clear
View Answer play_arrow
question_answer 249) Two stamens as exception in Cruciferae are found in:
A)
Nastrnsium
done
clear
B)
Senebiera
done
clear
C)
Raphanus
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 250) Difference between prokaryote and eukaryote is :
A)
in cell size
done
clear
B)
in cell shape
done
clear
C)
in chemical composition of protoplasm
done
clear
D)
in organization of nuclear material
done
clear
View Answer play_arrow
question_answer 251) Stomata are also called as:
A)
stomates
done
clear
B)
lenticels
done
clear
C)
hydathods
done
clear
D)
bark
done
clear
View Answer play_arrow
question_answer 252) In soil water available for plants is:
A)
capillary water
done
clear
B)
chemically bound water
done
clear
C)
gravitational water
done
clear
D)
hygroscopic water
done
clear
View Answer play_arrow
question_answer 253) Who discovered the super bugs?
A)
H.G. Khorana
done
clear
B)
Dilip Sah
done
clear
C)
Anand Mohan Chakrawarty
done
clear
D)
Robert
done
clear
View Answer play_arrow
question_answer 254) Genes are located in:
A)
chromosomes
done
clear
B)
ribosomes
done
clear
C)
lysosomes
done
clear
D)
sphaerosomes
done
clear
View Answer play_arrow
question_answer 255) In Amoeba, osmoregulation takes place by:
A)
vontractile vacuole
done
clear
B)
ectoplasm
done
clear
C)
pseudopodia
done
clear
D)
hyaloplasm
done
clear
View Answer play_arrow
question_answer 256) The infective stage of Plasmodium in humans is:
A)
sporozoite
done
clear
B)
trophozoite
done
clear
C)
gametocyte
done
clear
D)
merozoite
done
clear
View Answer play_arrow
question_answer 257) In Plasmodium ookinete is formed by:
A)
trophozoite
done
clear
B)
zygote
done
clear
C)
sporozoite
done
clear
D)
merozoite
done
clear
View Answer play_arrow
question_answer 258) In Plsmodium signet ring stage is formed during:
A)
exo-erythrocytic schizogony
done
clear
B)
erythrocytic schizogony
done
clear
C)
sporogony
done
clear
D)
gamogony
done
clear
View Answer play_arrow
question_answer 259) During unfavourable conditions Amoeba reproduces through:
A)
binary fission
done
clear
B)
sporulation
done
clear
C)
multiple fission
done
clear
D)
conjugation
done
clear
View Answer play_arrow
question_answer 260) Radial symmetry is found in:
A)
frog
done
clear
B)
starfish
done
clear
C)
humans
done
clear
D)
Pheretima
done
clear
View Answer play_arrow
question_answer 261) In Hydra reproduction occurs in favorable conditions :
A)
by budding
done
clear
B)
by gametes
done
clear
C)
by gemmules
done
clear
D)
binary fission
done
clear
View Answer play_arrow
question_answer 262) Haemocoel is present in:
A)
Pheretima
done
clear
B)
Periplaneta
done
clear
C)
Sponge
done
clear
D)
Ascaris
done
clear
View Answer play_arrow
question_answer 263) Chitin as exoskeleton found in:
A)
Periplaneta
done
clear
B)
Ascaris
done
clear
C)
Pheretima
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 264) Pseudocoel is present in:
A)
Periplaneta
done
clear
B)
Ascaris
done
clear
C)
Pheretima
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 265) Ascaris is found in:
A)
body cavity
done
clear
B)
lymph nodes
done
clear
C)
tissue
done
clear
D)
alimentary canal
done
clear
View Answer play_arrow
question_answer 266) Ascaris is:
A)
a parasite
done
clear
B)
a autotroph
done
clear
C)
facultative autotroph
done
clear
D)
facultative heterotrophy
done
clear
View Answer play_arrow
question_answer 267) Alimentary canal is absent in:
A)
Cestoda
done
clear
B)
Gastropoda
done
clear
C)
Nematoda
done
clear
D)
Pelecypoda
done
clear
View Answer play_arrow
question_answer 268) Metameric segmentation is the main feature of:
A)
Annelida
done
clear
B)
Echinodermata
done
clear
C)
Arthropoda
done
clear
D)
Coelenterata
done
clear
View Answer play_arrow
question_answer 269) Naked DNA without histones is found in:
A)
prokaryotes
done
clear
B)
eukaryotes
done
clear
C)
protozoa
done
clear
D)
coelenterate
done
clear
View Answer play_arrow
question_answer 270) Nematoblasts are formed by :
A)
interstitial cells
done
clear
B)
gland cells
done
clear
C)
mesoepithelial cells
done
clear
D)
nerve cells
done
clear
View Answer play_arrow
question_answer 271) Yellow spot is found in :
A)
muscles
done
clear
B)
nerves
done
clear
C)
kidney
done
clear
D)
eyes
done
clear
View Answer play_arrow
question_answer 272) Spindles are formed by:
A)
microtubules
done
clear
B)
endoplasmic reticulum
done
clear
C)
golgi body
done
clear
D)
peroxisomes
done
clear
View Answer play_arrow
question_answer 273) Scales are found in :
A)
pisces
done
clear
B)
rabbit
done
clear
C)
human
done
clear
D)
rat
done
clear
View Answer play_arrow
question_answer 274) Which is not correct for birds?
A)
Exothermic
done
clear
B)
Pneumatic bones
done
clear
C)
Lungs with air sacs
done
clear
D)
Amniotes
done
clear
View Answer play_arrow
question_answer 275) Which is not a part of hindbrain?
A)
Thalamus
done
clear
B)
Cerebellum
done
clear
C)
Pons
done
clear
D)
Medulla
done
clear
View Answer play_arrow
question_answer 276) Types of salivary glands present in rabbit are :
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 277) Which is not in pair in rabbit?
A)
Azygous vein
done
clear
B)
Hemizygous vein
done
clear
C)
Caudal vein
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 278) Canal system is present in phylum :
A)
Annelida
done
clear
B)
Porifera
done
clear
C)
Acanthocephala
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 279) Mitosis occurs in:
A)
skin cells
done
clear
B)
heart cells
done
clear
C)
kidney cells
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 280) Book lungs are the respiratory organs in:
A)
protozoans
done
clear
B)
cnidarians
done
clear
C)
arthropodes
done
clear
D)
amphibians
done
clear
View Answer play_arrow
question_answer 281) Ichthyophis belongs to:
A)
Amphibia
done
clear
B)
Reptilia
done
clear
C)
Pisces
done
clear
D)
Aves
done
clear
View Answer play_arrow
question_answer 282) Cleavage found in mesolecithal egg is:
A)
holoblastic and equal
done
clear
B)
holoblastic and unequal
done
clear
C)
meroblastic
done
clear
D)
discoidal
done
clear
View Answer play_arrow
question_answer 283) Egg of frog is:
A)
alecithal
done
clear
B)
macrolecithal
done
clear
C)
telolecithal
done
clear
D)
mesolecithal
done
clear
View Answer play_arrow
question_answer 284) Which is formed in gastrulation?
A)
Archenteron
done
clear
B)
Heart
done
clear
C)
Brain
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 285) Which is the function of nor-epinephrin ?
A)
Increase blood pressure
done
clear
B)
Urine formation
done
clear
C)
Increase secretion of adrenalin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 286) Ammonia is converted into urea in :
A)
kidney
done
clear
B)
lungs
done
clear
C)
liver
done
clear
D)
spleen
done
clear
View Answer play_arrow
question_answer 287) Vitamin C is present as :
A)
oxalic acid
done
clear
B)
glutemic acid
done
clear
C)
ascorbic acid
done
clear
D)
citric acid
done
clear
View Answer play_arrow
question_answer 288) Cortis organ is present in :
A)
Reisners membrane
done
clear
B)
Scala vestibule
done
clear
C)
Basilars membrane
done
clear
D)
Middle lamella
done
clear
View Answer play_arrow
question_answer 289) Eggs of cockroach give rise to:
A)
nymph
done
clear
B)
caterpillar
done
clear
C)
larva
done
clear
D)
pupa
done
clear
View Answer play_arrow
question_answer 290) Typhlosole is found in:
A)
Pheretima
done
clear
B)
Periplaneta
done
clear
C)
Hydra
done
clear
D)
Ascaris
done
clear
View Answer play_arrow
question_answer 291) Which is not an insect?
A)
Spider
done
clear
B)
Housefly
done
clear
C)
Cockroach
done
clear
D)
Mosquito
done
clear
View Answer play_arrow
question_answer 292) Echolocation system is found in:
A)
Equns
done
clear
B)
Amphibia
done
clear
C)
Bat
done
clear
D)
Oryctolagus
done
clear
View Answer play_arrow
question_answer 293) Which is a reptile?
A)
Salamander
done
clear
B)
Newt
done
clear
C)
Toad
done
clear
D)
Turtles
done
clear
View Answer play_arrow
question_answer 294) Covering of vacuoles is formed by :
A)
plasma membrane
done
clear
B)
tonoplast
done
clear
C)
leucoplast
done
clear
D)
symplast
done
clear
View Answer play_arrow
question_answer 295) Phenomenon of industrial melanism demonstrates :
A)
reproductive isolation
done
clear
B)
genetic isolation
done
clear
C)
natural selection
done
clear
D)
geographical isolation
done
clear
View Answer play_arrow
question_answer 296) Which sugar is present in nucleic acid?
A)
Pentose
done
clear
B)
Hexose
done
clear
C)
Fructose
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 297) Which of the following maintain the shape of cell?
A)
Osmotic pressure
done
clear
B)
Turgor pressure
done
clear
C)
Wall pressure
done
clear
D)
Osmosis
done
clear
View Answer play_arrow
question_answer 298) Vivipary is found in :
A)
Coelenterata
done
clear
B)
Protozoa
done
clear
C)
Rabbit
done
clear
D)
Pisces
done
clear
View Answer play_arrow
question_answer 299) The ultimate energy source of ecosystem is :
A)
solar energy
done
clear
B)
biomass
done
clear
C)
producer
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 300) Testis fail to descend in scrotal sacs, this condition is called as :
A)
cryptorchidism
done
clear
B)
hernia
done
clear
C)
castrection
done
clear
D)
none of these
done
clear
View Answer play_arrow