| (a) Write the steps involved in the extraction of pure metals in middle of the activity series from carbonate ores. |
| (b) How is copper extracted from its sulphide ore? Explain the various steps supported by chemical equations. Draw labelled diagram for electrolytic refining of copper. |
Answer:
(a) First of all the carbonate ore of a metal is heated in absence of air. This process is called calcination
\[\underset{\text{Zinc Carbonate}}{\mathop{ZnC{{O}_{3}}}}\,\xrightarrow[\text{absence of air}]{\text{Heat}}\underset{\text{Zinc oxide}}{\mathop{ZnO}}\,\mathrm{ }+\underset{\text{Carbon dioxide}}{\mathop{C{{O}_{2}}}}\,\]
The, \[ZnO\] is heated with coke.
\[\underset{{}}{\mathop{ZnO}}\,+\underset{Coke}{\mathop{C}}\,\to \underset{Pure\mathrm{ }metal}{\mathop{Zn}}\,+\underset{Carbon\mathrm{ }monoxide}{\mathop{CO}}\,\]
(b) Copper is extracted from sulphide ore by the process of roasting. It is done in presence of air:
\[2C{{u}_{2}}S+3{{O}_{2}}\xrightarrow{Heat}C{{u}_{2}}O+2S{{O}_{2}}\]
\[2C{{u}_{2}}O+C{{u}_{2}}S\xrightarrow{Heat}6Cu+\mathrm{ }2S{{O}_{2}}\]
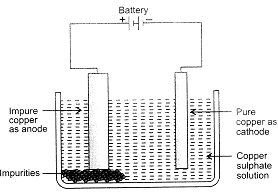
Electrolytic Refining of Copper:
You need to login to perform this action.
You will be redirected in
3 sec
