question_answer 1) A simple pendulum of length \[l\] has a maximum angular displacement \[\text{ }\!\!\theta\!\!\text{ }\]. The maximum kinetic energy of the bob is :
A)
\[mgl\]\[(1-cos\theta )\]
done
clear
B)
0.5\[mgl\]
done
clear
C)
\[mgl\]
done
clear
D)
0.5 \[mgl\]
done
clear
View Answer play_arrow
question_answer 2) Radius of orbit of satellite of earth is R. Its kinetic energy is proportional to :
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{\sqrt{R}}\]
done
clear
C)
\[R\]
done
clear
D)
\[\frac{1}{{{R}^{3/2}}}\]
done
clear
View Answer play_arrow
question_answer 3) The radius R of the soap bubble is doubled under isothermal condition. If T be the surface tension of soap bubble. The work done in doing so is given by :
A)
\[32{{\pi }^{2}}T\]
done
clear
B)
\[24\pi {{R}^{2}}T\]
done
clear
C)
\[8\pi {{R}^{2}}T\]
done
clear
D)
\[4\pi {{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 4) A body of specific heat\[0.2\text{ }kcal/kg{}^\circ C\]is heated through 1000C. The percentage increase in its mass is :
A)
9%
done
clear
B)
\[9.3\times {{11}^{-11}}%\]
done
clear
C)
10%
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 5) Two similar coils are kept mutually perpendicular such that their centres coincide. At the centre, find the ratio of the magnetic field due to one coil and the resultant magnetic field through both coils, if the same current is flows:
A)
\[1:\sqrt{2}\]
done
clear
B)
1 : 2
done
clear
C)
1 : 2
done
clear
D)
\[\sqrt{3}:1\]
done
clear
View Answer play_arrow
question_answer 6) A prism of refractive index \[\sqrt{2}\] has a refracting angle of\[60{}^\circ \]. At what angle a ray must be incident on it, so that it suffers a minimum deviation :
A)
\[45{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[180{}^\circ \]
done
clear
View Answer play_arrow
question_answer 7) A cone filled with water is revolved in a vertical circle of radius 4 m and the water does not fall down. What must be the maximum period of revolution?
A)
2 s
done
clear
B)
4 s
done
clear
C)
1s
done
clear
D)
6 s
done
clear
View Answer play_arrow
question_answer 8) A conducting sphere of radius R = 20 cm is given a charge \[Q\]= 16\[\mu \]C. What is \[\hat{E}\] at centre?
A)
\[3.6\times {{10}^{6}}\,N/C\]
done
clear
B)
\[1.8\times {{10}^{6}}\,N/C\]
done
clear
C)
Zero
done
clear
D)
\[0.9\times {{10}^{6}}\,N/C\]
done
clear
View Answer play_arrow
question_answer 9) Three circular concentric wires of radii a, \[2a\] and \[3a\] are carrying current \[3l,2l\] and \[l\] in same manner. The magnetic field at the common centre is:
A)
\[\frac{13{{\mu }_{0}}l}{6a}\]
done
clear
B)
\[\frac{{{\mu }_{0}}l}{6a}\]
done
clear
C)
\[\frac{{{\mu }_{0}}l}{a}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 10) The maximum range of a gun on horizontal terrain is 16 km, if\[g=10\text{ }m/{{s}^{2}}\]. What must be the muzzle velocity of the shell?
A)
200 m/s
done
clear
B)
100 m/s
done
clear
C)
400 m/s
done
clear
D)
300 m/s
done
clear
View Answer play_arrow
question_answer 11) The length, breadth and thickness of a block are given by \[l\] = 12 cm, b = 6 cm and t = 2.45 cm. The volume of the block according to the idea of significant figures should be:
A)
\[1\times {{10}^{2}}c{{m}^{3}}\]
done
clear
B)
\[2\times {{10}^{2}}c{{m}^{3}}\]
done
clear
C)
\[1.763\times {{10}^{2}}c{{m}^{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 12) Five particles of mass 2 kg are attached to the rim of a circular disc of radius 0.1 m and negligible mass. Moment of inertia of the system about the axis passing through the centre of the disc and perpendicular to its plane is :
A)
\[1\text{ }kg\text{ }{{m}^{2}}\]
done
clear
B)
\[0.1\text{ }kg\text{ }{{m}^{2}}\]
done
clear
C)
\[2\text{ }kg\text{ }{{m}^{2}}\]
done
clear
D)
\[0.2\text{ }kg\text{ }{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 13) The radius of the convex surface of plano-convex lens is 20 cm and the refractive index of the material of the lens is 1.5. The focal length is:
A)
30 cm
done
clear
B)
50 cm
done
clear
C)
20 cm
done
clear
D)
40 cm
done
clear
View Answer play_arrow
question_answer 14) An ice-cube of density\[900\text{ }kg/{{m}^{3}}\]is floating in water of density\[1000\text{ }kg/{{m}^{3}}\]. The percentage of Volume of ice-cube outside the water is:
A)
20%
done
clear
B)
35%
done
clear
C)
10%
done
clear
D)
25%
done
clear
View Answer play_arrow
question_answer 15) A sphere of diameter 0.2 m and mass 2 kg is rolling on an inclined plane with velocity\[v=0.5\]m/s. The kinetic energy of the sphere is :
A)
0.1 J
done
clear
B)
0.3 J
done
clear
C)
0.5 J
done
clear
D)
0.42 J
done
clear
View Answer play_arrow
question_answer 16) An electron moves at right angle to a magnetic field of\[5\times {{10}^{-2}}T\]with a speed of\[6\times {{10}^{7}}m/s\]. If the specific charge of the electron is\[1.7\times {{10}^{11}}\] C/kg. The radius of the circular path will be:
A)
2.9 cm
done
clear
B)
3.9 cm
done
clear
C)
2.35 cm
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 17) If work function of a metal is 4.2eV, the cut off wavelength is:
A)
\[8000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[7000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1472\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2950\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 18) A particle is executing the motion\[x=a\,\cos (\omega t-\text{ }\!\!\theta\!\!\text{ }).\]\[\text{( }\!\!\omega\!\!\text{ t- }\!\!\theta\!\!\text{ )}\text{.}\]). The velocity of the particle is:
A)
\[a\omega \,\sin \text{ }\!\!\theta\!\!\text{ }\]
done
clear
B)
\[a\omega \,\]
done
clear
C)
\[a\omega \,\,\sin \text{ }\!\!\theta\!\!\text{ }\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) A particle is executing two different simple harmonic motions, mutually perpendicular, of different amplitudes and having phas difference of \[\frac{\pi }{2}.\]The path of the particle will be:
A)
circular
done
clear
B)
straight line
done
clear
C)
paraboilic
done
clear
D)
elliptical
done
clear
View Answer play_arrow
question_answer 20) Equations of motion in the same direction are given by: \[{{y}_{1}}=2a\sin (\omega t-kx)\] \[{{y}_{2}}=2a\sin (\omega t-kx-\text{ }\!\!\theta\!\!\text{ })\] The amplitude of the medium particle will be:
A)
\[2a\sin \text{ }\!\!\theta\!\!\text{ }\]
done
clear
B)
\[\sqrt{2}a\sin \text{ }\!\!\theta\!\!\text{ }\]
done
clear
C)
\[4a\sin \frac{\text{ }\!\!\theta\!\!\text{ }}{2}\]
done
clear
D)
\[\sqrt{2}a\sin \frac{\text{ }\!\!\theta\!\!\text{ }}{2}\]
done
clear
View Answer play_arrow
question_answer 21) The work function of sodium is 2.3 eV. The threshold wavelength of sodium will be:
A)
\[2900\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2500\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[5380\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1200\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
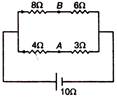
question_answer 22)
The potential difference between points A and B is :
A)
\[\frac{20}{7}V\]
done
clear
B)
\[\frac{40}{7}V\]
done
clear
C)
\[\frac{10}{7}V\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 23) The velocity of light emitted by a source S observed by an observer at rest w.r.t S is c. If he observer moves with a speed v towards S, then velocity of light as observed by the observer will be:
A)
c
done
clear
B)
\[\sqrt{1-{{v}^{4}}{{c}^{2}}}\]
done
clear
C)
\[c+v\]
done
clear
D)
c - v
done
clear
View Answer play_arrow
question_answer 24) A closed argon pipe and an open argon pipe are tuned to the same fundamental frequency. What is the ratio of their lengths?
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
2 : 3
done
clear
D)
4 : 3
done
clear
View Answer play_arrow
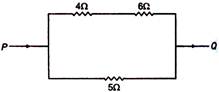
question_answer 25)
When a certain current is passed in the circuit as shown in figure, 10 kcal of heat is produced in 5\[\Omega \] resistance. How much heat is produced in \[\Omega \] resistance?
A)
4 kcal
done
clear
B)
2 kcal
done
clear
C)
5 kcal
done
clear
D)
3 kcal
done
clear
View Answer play_arrow
question_answer 26) A steel scale measures the length of a copper wire as 80.0 cm, when both are at\[20{}^\circ C,\] the calibration temperature for the scale. What would the scale read for the length of the rod when both are at\[40{}^\circ C\]? Given: a for steel \[=11\times {{10}^{-6}}per{}^\circ C\] and \[a\] for \[Cu=17\times {{10}^{-6}}per{}^\circ C\]
A)
80.0096 cm
done
clear
B)
80.0272 cm
done
clear
C)
1 cm
done
clear
D)
25.2 cm
done
clear
View Answer play_arrow
question_answer 27) A tank is filled with water upto height H. When hole is made at a distance h below the level of water. What will be the horizontal range of water jet?
A)
\[2\sqrt{h(H-h)}\]
done
clear
B)
\[4\sqrt{h(H+h)}\]
done
clear
C)
\[4\sqrt{h(H+h)}\]
done
clear
D)
\[2\sqrt{h(H+h)}\]
done
clear
View Answer play_arrow
question_answer 28) A raft of wood of mass 120 kg floats in water. The weight that can be put on the raft to make it just sink, should be \[{{d}_{raft}}=600kg/{{m}^{3}}\]:
A)
80 kg
done
clear
B)
50 kg
done
clear
C)
60 kg
done
clear
D)
30 kg
done
clear
View Answer play_arrow
question_answer 29) A particle is kept at rest at the top of a sphere of diameter 42 m. When disturbed slightly, it slides down. At what height h from the bottom, the particle will leave the sphere?
A)
14 m
done
clear
B)
28 m
done
clear
C)
35 m
done
clear
D)
7 m
done
clear
View Answer play_arrow
question_answer 30) If an insulated non-conducting sphere of radius R has charge density p. The electric field at a distance r from the centre of sphere (r > R) will
A)
\[\frac{\rho R}{3{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{\rho r}{{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{\rho r}{3{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{3\rho R}{{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
question_answer 31) The minimum wavelength of X-ray emitted by X-ray tube is\[0.4125\overset{o}{\mathop{\text{A}}}\,\]. The accelerating voltage is:
A)
30 kV
done
clear
B)
50 kV
done
clear
C)
80 kV
done
clear
D)
60 kV
done
clear
View Answer play_arrow
question_answer 32) A monoatomic gas supplied the heat \[Q\] very slowly keeping the pressure constant. The work done by the gas will be :
A)
\[\frac{2}{3}Q\]
done
clear
B)
\[\frac{3}{5}Q\]
done
clear
C)
\[\frac{2}{5}Q\]
done
clear
D)
\[\frac{1}{5}Q\]
done
clear
View Answer play_arrow
question_answer 33) A thin lens has focal length \[f\] and its aperture has diameter d. It forms an image of intensity \[l\]. Now the central part of the aperture upto diameter \[d/\] 2 is blocked up by an opaque paper. The focal length and the image intensity will change to :
A)
\[\frac{f}{2}and\frac{I}{2}\]
done
clear
B)
\[f\,and\frac{I}{4}\]
done
clear
C)
\[\frac{3f}{4}\,and\frac{I}{2}\]
done
clear
D)
\[f\,and\frac{3I}{4}\]
done
clear
View Answer play_arrow
question_answer 34) The temperature of the black body increases from T to 2T. The factor by which the rate of emission will increase, is?
A)
4
done
clear
B)
2
done
clear
C)
16
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 35) A police jeep is chasing with velocity of 45 km/h a thief in a nother jeep moving with velocity 153 km/h. Police fires a bullet with muzzle velocity of 180 m/s. The velocity it will-strike the car of the thief is :
A)
150 m/s
done
clear
B)
27 m/s
done
clear
C)
450 m/s
done
clear
D)
250 m/s
done
clear
View Answer play_arrow
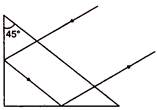
question_answer 36)
What should be the minimum value of refractive index of the material of the prism for the reflections to take place as shown in the figure :
A)
1.7
done
clear
B)
1.4
done
clear
C)
1.2
done
clear
D)
2.7
done
clear
View Answer play_arrow
question_answer 37) An \[LC\] circuit is in the state of resonance. If C = 0.1 \[\mu F\]and L = 0.25 H. Neglecting ohmic resistance of circuit. What is the frequency of oscillations?
A)
1007 Hz
done
clear
B)
100 Hz
done
clear
C)
109 Hz
done
clear
D)
500 Hz
done
clear
View Answer play_arrow
question_answer 38) A person who can see things most clearly at a distance of 10 cm, requires spectacles to enable to see clearly things at a distance of 30 cm. What should be the focal length of the spectacles?
A)
15 cm (Concave)
done
clear
B)
15 cm (Convex)
done
clear
C)
10 cm
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 39) The dimensional formula for Youngs modulus is:
A)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
B)
\[[{{M}^{0}}L{{T}^{-2}}]\]
done
clear
C)
\[[ML{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 40) When temperature of an ideal gas is increased from\[27{}^\circ C\]to\[227{}^\circ C,\] its rms speed is changed from 400 m/s to \[{{v}_{s}}\] The \[{{v}_{s}}\] is :
A)
516 m/s
done
clear
B)
450 m/s
done
clear
C)
310 m/s
done
clear
D)
746 m/s
done
clear
View Answer play_arrow
question_answer 41) Graphite is soft while diamond is hard because:
A)
graphite is in powder form
done
clear
B)
diamond has\[s{{p}^{2}}\]hybridization but graphite has\[s{{p}^{3}}\]hybridization
done
clear
C)
graphite is in planar form while diamond is in tetrahedral form
done
clear
D)
graphite is covalent and diamond is ionic
done
clear
View Answer play_arrow
question_answer 42) The pH of a\[{{10}^{-10}}M\,NaOH\]solution is:
A)
10
done
clear
B)
7.01
done
clear
C)
6.99
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 43) \[N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{{I}_{2}}\xrightarrow[{}]{{}}\]Product is:
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[N{{a}_{2}}{{S}_{4}}{{O}_{6}}\]
done
clear
D)
\[{{S}_{2}}\]
done
clear
View Answer play_arrow
question_answer 44) Phenol and benzoic acid are distinguished by:
A)
\[NaOH\]
done
clear
B)
\[NaHC{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 45) \[{{H}_{2}}O\]is liquid while\[{{H}_{2}}S\]is a gas due to:
A)
covalent bonding
done
clear
B)
molecular attraction
done
clear
C)
H-bonding
done
clear
D)
both H-bonding and molecular attraction
done
clear
View Answer play_arrow
question_answer 46) Phenol reacts with chloroform in presence of:
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[KOH\]
done
clear
C)
\[NaN{{O}_{2}}/HCl\]
done
clear
D)
Grignard reagent
done
clear
View Answer play_arrow
question_answer 47) Frenkel and Schottky defects are:
A)
nucleus defects
done
clear
B)
non-crystal defects
done
clear
C)
crystal defects
done
clear
D)
nuclear defects
done
clear
View Answer play_arrow
question_answer 48) \[C{{H}_{3}}C{{H}_{2}}C\equiv CH\xrightarrow[{}]{R}\]butanone-2. R is:
A)
\[H{{g}^{2+}}/dil.{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[KMn{{O}_{4}}\]
done
clear
C)
\[KCl{{O}_{3}}\]
done
clear
D)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 49) Which has minimum solubility?
A)
\[B{{i}_{2}}{{S}_{3}}\]
done
clear
B)
\[A{{g}_{2}}S\]
done
clear
C)
\[CoS\]
done
clear
D)
\[PbS\]
done
clear
View Answer play_arrow
question_answer 50) Ratio of molecular weights of A and B is\[\frac{4}{25},\]then ratio of rates of diffusion will be:
A)
\[5:1\]
done
clear
B)
\[5:2\]
done
clear
C)
\[25:3\]
done
clear
D)
\[25:4\]
done
clear
View Answer play_arrow
question_answer 51) H-bond is strongest in:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[H-F\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 52) Strongest nucleophile is:
A)
\[RN{{H}_{2}}\]L
done
clear
B)
ROH
done
clear
C)
\[{{C}_{6}}{{H}_{5}}{{O}^{-}}\]
done
clear
D)
\[R{{H}_{3}}{{O}^{-}}\]
done
clear
View Answer play_arrow
question_answer 53) \[a/{{V}^{2}}\]given in van der Waals, equation is for:
A)
internal pressure
done
clear
B)
intermolecular attraction
done
clear
C)
both [a] and [b]
done
clear
D)
temperature correction
done
clear
View Answer play_arrow
question_answer 54) The hardest substance is:
A)
iron
done
clear
B)
steel
done
clear
C)
diamond
done
clear
D)
graphite
done
clear
View Answer play_arrow
question_answer 55) Which of the following is wrong statement?
A)
\[Ni{{(CO)}_{4}}\]has oxidation number +4 for \[Ni\]
done
clear
B)
\[Ni{{(CO)}_{4}}\]has zero oxidation number for\[Ni\]
done
clear
C)
\[Ni\]is metal
done
clear
D)
CO is gas
done
clear
View Answer play_arrow
question_answer 56) Hybridization of 1 and 2 carbon atom in\[C{{H}_{2}}=C=C{{H}_{2}}\]are:
A)
\[sp,\text{ }sp\]
done
clear
B)
\[s{{p}^{2}},\text{ }s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}},\text{ }sp\]
done
clear
D)
\[s{{p}^{3}},\text{ }s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 57) In\[{{[NiC{{l}_{4}}]}^{2-}},\]the number of unpaired electrons, are:
A)
4
done
clear
B)
2
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 58) Which carbocation is most stable?
A)
\[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
B)
\[\overset{+}{\mathop{C}}\,{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 59) \[C{{H}_{3}}\overset{\begin{smallmatrix} C{{H}_{2}}C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C{{H}_{3}}}}\,\xrightarrow[{{H}_{2}}]{catalyst}X,\] Number of optical isomer possible will be:
A)
2
done
clear
B)
4
done
clear
C)
0
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 60) \[C(dia)+{{O}_{2}}\xrightarrow[{}]{{}}C{{O}_{2}};\]\[\Delta H=-395.4\text{ }kJ/mol\] \[C(gr)+{{O}_{2}}\xrightarrow[{}]{{}}C{{O}_{2}}\] \[\Delta H=-393.5\text{ }kJ/mol\] \[C(gr)\xrightarrow[{}]{{}}C(dia);\Delta H=?:\]
A)
\[-3.8\]
done
clear
B)
\[-1.9\]
done
clear
C)
\[+3.8\]
done
clear
D)
\[+1.9\]
done
clear
View Answer play_arrow
question_answer 61) The internal energy of a substance:
A)
decrease with increase in temperature
done
clear
B)
increase with increase in temperature
done
clear
C)
remains unaffected with change in temperature
done
clear
D)
calculated by \[E=m{{c}^{2}}\]
done
clear
View Answer play_arrow
question_answer 62) The rate constant of a reaction depends on:
A)
the temperature of a reaction
done
clear
B)
the time of a reaction
done
clear
C)
the extent of reaction
done
clear
D)
the initial concentration of the reactant
done
clear
View Answer play_arrow
question_answer 63) The half life of a radioactive element is 40 days. Calculate the average life.
A)
5.76 days
done
clear
B)
57.6 days
done
clear
C)
646 days
done
clear
D)
4.56 days
done
clear
View Answer play_arrow
question_answer 64) In an isothermal process:
A)
\[\Delta H=\Delta E+P\Delta V\]
done
clear
B)
\[\Delta H=W\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
\[\Delta H=S\Delta T\]
done
clear
View Answer play_arrow
question_answer 65) Number of orbitals in L energy level:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 66) How much chlorine will be liberated on passing one ampere current for 30 minute through\[NaCl\]solution?
A)
0.66 mole
done
clear
B)
0.33 mole
done
clear
C)
0.66g
done
clear
D)
0.33g
done
clear
View Answer play_arrow
question_answer 67) \[{{H}_{2}}{{C}_{2}}{{O}_{4}}+KMn{{O}_{4}}\]in acidic medium reacts to have change in oxidation number of Mn from:
A)
7 to 5
done
clear
B)
2 to 7
done
clear
C)
5 to 7
done
clear
D)
7 to 2
done
clear
View Answer play_arrow
question_answer 68) When the temperature of an ideal gas is increased from\[27{}^\circ C\]to\[927{}^\circ C,\]the kinetic energy will be:
A)
same
done
clear
B)
eight times
done
clear
C)
four times
done
clear
D)
twice
done
clear
View Answer play_arrow
question_answer 69) Ratio of radii of second and first Bohr orbits of H atom is:
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 70) India conducted an underground nuclear test at:
A)
Narora
done
clear
B)
Tarapur
done
clear
C)
Pushkar
done
clear
D)
Pokharan
done
clear
View Answer play_arrow
question_answer 71) Which is used as medicine?
A)
PVC
done
clear
B)
Terylene
done
clear
C)
Glyptal
done
clear
D)
Urotropine
done
clear
View Answer play_arrow
question_answer 72) \[NaOH/{{H}^{+}}\]reacts with:
A)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,C{{H}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 73) Butyne-land butyne-2 can be distinguished by
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[AgN{{O}_{3}}+N{{H}_{4}}OH\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
D)
\[N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 74) The incorrect configuration is:
A)
\[K=[Ar]\text{ }4{{s}^{1}}\]
done
clear
B)
\[Cr=[Ar]\text{ }3{{d}^{5}},4{{s}^{1}}\]
done
clear
C)
\[Cr=[Ar]\text{ }3{{d}^{4}},\text{ }4{{s}^{2}}\]
done
clear
D)
\[Cu=[Ar]\text{ }3{{d}^{10}},4{{s}^{1}}\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following compound gives iodoform test?
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 76) Dual nature of particle was given by:
A)
Bohr theory
done
clear
B)
Thomson model
done
clear
C)
Heisenberg principle
done
clear
D)
de-Broglie equation
done
clear
View Answer play_arrow
question_answer 77) \[NaOH(aq),\text{ }HCl(aq),\]and\[NaCl(aq)\]concentration of each is \[{{10}^{-3}}M\]. Their pH will be respectively:
A)
10, 6, 2
done
clear
B)
11, 3, 7
done
clear
C)
10, 3, 7
done
clear
D)
10, 4, 7
done
clear
View Answer play_arrow
question_answer 78) Most acidic is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHCOOH\]
done
clear
C)
\[HCOOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 79) Substance having ester linkage is:
A)
bakelite
done
clear
B)
polythene
done
clear
C)
PVC
done
clear
D)
terylene
done
clear
View Answer play_arrow
question_answer 80) Carborandum is:
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[CuC{{O}_{3}}\]
done
clear
D)
\[SiC\]
done
clear
View Answer play_arrow
question_answer 81) Which one of the following is the most polar bond?
A)
\[N-F\]
done
clear
B)
\[N-N\]
done
clear
C)
\[N-Cl\]
done
clear
D)
\[O-F\]
done
clear
View Answer play_arrow
question_answer 82) Which one of the following molecules does not exist?
A)
\[H{{e}_{2}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) 1 mole. of cone. HCl requires x moles of dil. \[NaOH\]for neutralisation and 1 mole of cone. \[{{H}_{2}}S{{O}_{4}}\]requires y moles of small dil.\[HCl\]. Then which of the following equation is true?
A)
\[y=\frac{1}{2}x\]
done
clear
B)
\[x=\frac{1}{2}y\]
done
clear
C)
\[x=2y\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) An ideal gas expands in volume from \[1\times {{10}^{-3}}{{m}^{3}}\]to\[1\times {{10}^{-2}}{{m}^{3}}\]at 300 K against a constant pressure of\[1\times {{10}^{5}}N{{m}^{-2}}\]. The work done is:
A)
\[-900J\]
done
clear
B)
\[-900kJ\]
done
clear
C)
\[270\text{ }kJ\]
done
clear
D)
\[900\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 85) When IF of electricity is passed through acidulated water,\[{{O}_{2}}\]evolved is:
A)
\[11.2\text{ }d{{m}^{3}}\]
done
clear
B)
\[5.6\text{ }d{{m}^{3}}\]
done
clear
C)
\[22.4\text{ }d{{m}^{3}}\]
done
clear
D)
\[1.0\text{ }d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 86) Freundlich adsorption isotherm is:
A)
\[\frac{x}{m}=k{{p}^{1/n}}\]
done
clear
B)
\[x=mk{{p}^{1/n}}\]
done
clear
C)
\[\frac{x}{m}=k{{p}^{-n}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 87) Conjugate base of\[HS{{O}_{4}}\]is:
A)
\[SO_{4}^{2-}\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{H}_{3}}SO_{4}^{+}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 88) pH of\[1\times {{10}^{-8}}M\text{ }HN{{O}_{3}}\]solution will be:
A)
6
done
clear
B)
6.9586
done
clear
C)
7.9586
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 89) The moderator used in nuclear reactor is:
A)
TEL
done
clear
B)
\[{{D}_{2}}O\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[R-O-P\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following is used in the treatment of blood cancer?
A)
\[{{I}^{131}}\]
done
clear
B)
\[{{P}^{32}}\]
done
clear
C)
\[R{{a}^{226}}\]
done
clear
D)
\[{{I}^{127}}\]
done
clear
View Answer play_arrow
question_answer 91) Formula of felspar is:
A)
\[{{K}_{2}}O.A{{l}_{2}}{{O}_{3}}.6Si{{O}_{2}}\]
done
clear
B)
\[{{K}_{2}}{{O}_{3}}.A{{l}_{2}}{{O}_{3}}.6S{{i}_{2}}{{O}_{2}}.2{{H}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}.2Si{{O}_{2}}.2{{H}_{2}}O\]
done
clear
D)
\[3MgO.4Si{{O}_{2}}.{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 92) \[A{{l}_{2}}{{O}_{3}}\]can be reduced by:
A)
Mg
done
clear
B)
\[Si\]
done
clear
C)
S
done
clear
D)
\[Cl\]
done
clear
View Answer play_arrow
question_answer 93) Best method of preparing alkyl chloride is:
A)
\[ROH+SOC{{l}_{2}}\xrightarrow[{}]{{}}\]
done
clear
B)
\[ROH+PC{{l}_{5}}\xrightarrow[{}]{{}}\]
done
clear
C)
\[ROH+PC{{l}_{3}}\xrightarrow[{}]{{}}\]
done
clear
D)
\[ROH+HCl\xrightarrow[{}]{anhy.\,ZnC{{l}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 94) Chloropicrin is used as:
A)
solvent
done
clear
B)
anaesthetic
done
clear
C)
perfume
done
clear
D)
tear gas
done
clear
View Answer play_arrow
question_answer 95) Propyl amine on treatment with nitrous acid gives:
A)
ethanol
done
clear
B)
isopropyi alcohol
done
clear
C)
propanol
done
clear
D)
propanol
done
clear
View Answer play_arrow
question_answer 96) On strongly heating ammonium acetate gives:
A)
acetamide
done
clear
B)
methyl cyanide
done
clear
C)
urea
done
clear
D)
formamide
done
clear
View Answer play_arrow
question_answer 97) The main structural feature of proteins is:
A)
ester linkage
done
clear
B)
ether linkage
done
clear
C)
peptide linkage
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 98) Among the following a natural polymer is:
A)
cellulose
done
clear
B)
PVC
done
clear
C)
teflon
done
clear
D)
polyethylene
done
clear
View Answer play_arrow
question_answer 99) Glucose shows reducing nature because it has a:
A)
aldehydic group
done
clear
B)
ketone group
done
clear
C)
hydroxyl group
done
clear
D)
\[-N{{H}_{2}}\]group
done
clear
View Answer play_arrow
question_answer 100) In the following reaction, X is: \[X\xrightarrow[{}]{bromination}Y\xrightarrow[+HCl]{NaN{{O}_{2}}}\] \[Z\xrightarrow[{{C}_{2}}{{H}_{5}}OH]{Boiling}Tribromo\text{ }benzene\]
A)
benzoic acid
done
clear
B)
salicylic acid
done
clear
C)
phenol
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 101) In uremia, artificial kidney is used for removing accumulated waste products like urea by the process, called :
A)
maturation
done
clear
B)
ureotelism
done
clear
C)
reverse dialysis
done
clear
D)
haemodialysis
done
clear
View Answer play_arrow
question_answer 102) Mesophyll is differentiated into palisade and spongy parenchyma is adaptation to :
A)
light intensity
done
clear
B)
reduced tanspiration
done
clear
C)
low water availability
done
clear
D)
atmospheric humidity
done
clear
View Answer play_arrow
question_answer 103) Bulliform cells that help in the following down of lamina in drought, are present in epidermis of :
A)
monocotyledonous grass leaf
done
clear
B)
dicotyledonous leaf
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 104) Which of the following apparatus is commonly used to measure the rate of transpiration is ?
A)
Porometer
done
clear
B)
Altimeter
done
clear
C)
Potometer
done
clear
D)
Luxmeter
done
clear
View Answer play_arrow
question_answer 105) Which one of the following contains copper besides iron?
A)
Cytochrome-\[f\]
done
clear
B)
Cytochrome oxidase
done
clear
C)
Cytochrome-\[{{b}_{2}}\]
done
clear
D)
Cytochrome-\[{{c}_{1}}\]
done
clear
View Answer play_arrow
question_answer 106) Genetically dwarf plant can be converted into a plant of normal height with the application of:
A)
ethylene
done
clear
B)
gibberellins
done
clear
C)
cytokinins
done
clear
D)
auxin
done
clear
View Answer play_arrow
question_answer 107) A structure known as peroxisomes is associated with :
A)
photosynthesis
done
clear
B)
respiration
done
clear
C)
photorespiration
done
clear
D)
photo phosphorylation
done
clear
View Answer play_arrow
question_answer 108) A phytohormone involved in the de novo synthesis of a-amylase in germinating seeds is:
A)
auxin
done
clear
B)
gibberellin
done
clear
C)
ethylene
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 109) Which of the following element is most mobile in plant metabolism ?
A)
Calcium
done
clear
B)
Phosphorus
done
clear
C)
Carbon
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 110) For amino-transferases the essential coenzyme is :
A)
Co-A
done
clear
B)
Pyridoxyl phosphate
done
clear
C)
DPN
done
clear
D)
NAD
done
clear
View Answer play_arrow
question_answer 111) The process of converting ammonid to nitrate by bacteria is known as :
A)
ammonification
done
clear
B)
nitrification
done
clear
C)
nitrogen fixation
done
clear
D)
denitrification
done
clear
View Answer play_arrow
question_answer 112) In the mesophyll cells of CAM plants, \[C{{O}_{2}}\] fixation during day occur through :
A)
RuBP-oxygenase
done
clear
B)
PEP-carboxylase
done
clear
C)
RuBP-carboxylase
done
clear
D)
Both RuBP carboxylase and PEP-carboxylase
done
clear
View Answer play_arrow
question_answer 113) Various changes in mammalian sperm which prepare it to fertilise the ovum is called :
A)
capacitation
done
clear
B)
regeneration
done
clear
C)
growth
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 114) Sometimes a gene masks the expression of another gene at a different locus. This phenomenon is known as :
A)
codominance
done
clear
B)
epistasis
done
clear
C)
incomplete dominance
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 115) In the F, generation of any attempted cross when neither the gene for red nor white is dominant or recessive. In this case, both the genes express them-selfes partially the phenomenon is known as :
A)
pseudo dominance
done
clear
B)
dominance
done
clear
C)
codominance
done
clear
D)
incomplete dominance
done
clear
View Answer play_arrow
question_answer 116) In order to explain the mode of inheritance of character through successive generations Mendel proposed that the two alternative factors for each character become separated during the formation of gametes and each factors has an equal chance of being transferred the off springs. This phenomenon is known as :
A)
principle of independent assortment
done
clear
B)
principle of segregation
done
clear
C)
principle of incomplete dominance
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 117) The diagrammetic representation of a chromosome is known as :
A)
idiotype
done
clear
B)
karyotype
done
clear
C)
homodyne
done
clear
D)
holotype
done
clear
View Answer play_arrow
question_answer 118) Thread like structure that are composed of the nuclear DNA of eukaryotic cells and are the carrier of genetic information. These structure were known as chromosomes. The term chromosomes was given by :
A)
Waldeyer
done
clear
B)
Balbiani
done
clear
C)
Purkinje
done
clear
D)
Sutton
done
clear
View Answer play_arrow
question_answer 119) A trihybrid cross involve three pair of characters which will give rise to the \[{{\text{F}}_{\text{1}}}\]hybrids which are heterozygous for three genes. How many types of gametes will be produced in both male and female ?
A)
2
done
clear
B)
4
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 120) A functional complex comprising a cluster of genes including structural gene, a promoter gene, an operator region and a regulatory gene was discovered by :
A)
Beadle and Tatum (1958)
done
clear
B)
Watson and Crick (1953)
done
clear
C)
Tacob and Monad (1961)
done
clear
D)
Britten and Davidson (1969)
done
clear
View Answer play_arrow
question_answer 121) Which one of the following traits studied by Mendel in garden pea was a recessive character?
A)
Axial flower position
done
clear
B)
Green seed colour
done
clear
C)
Green pod colour
done
clear
D)
Pound seed colour
done
clear
View Answer play_arrow
question_answer 122) When an \[{{\text{F}}_{\text{1}}}\] individuals is crossed with its either of the two parent. Then it is known as :
A)
test cross
done
clear
B)
back cross
done
clear
C)
reciprocal cross
done
clear
D)
monohybrid cross
done
clear
View Answer play_arrow
question_answer 123) Which one of the following species yield edible oil and fibre ?
A)
Cocos nucifera
done
clear
B)
Brassica compestris
done
clear
C)
Mangifera indica
done
clear
D)
Arachis hypogea
done
clear
View Answer play_arrow
question_answer 124) Botanical name of arhu is :
A)
Prunus amygdalus
done
clear
B)
Prunus armeniaca
done
clear
C)
Prunus persica
done
clear
D)
Prunus avium
done
clear
View Answer play_arrow
question_answer 125) Out of following given cereals which is a pseudocereals ?
A)
Sorghum
done
clear
B)
Millets
done
clear
C)
Buckwheat
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 126) State which of the following statement is not associated with the use of ginger ?
A)
Used as condiment
done
clear
B)
Used in digestive disorder
done
clear
C)
Suitable for making starch
done
clear
D)
Distilled oil is used in perfurmery
done
clear
View Answer play_arrow
question_answer 127) Telocentric chromosomes differ from acrocentric chromosomes in having :
A)
terminal centromere as compared to subterminal centromere in the latter
done
clear
B)
terminal centromere as compared to medianly located centromere in acrocentric
done
clear
C)
subterminal centromere as compared to medianly located centromere in acrocentric
done
clear
D)
subterminal centromere as compared to submedian centromere of acrocentric one
done
clear
View Answer play_arrow
question_answer 128) Chromosomes present in prolonged prophase in the salivary glands of Drosophila are :
A)
polytene
done
clear
B)
B-chromosomes
done
clear
C)
Lampbrush
done
clear
D)
supernumerary chromosomes
done
clear
View Answer play_arrow
question_answer 129) Chromosomes at anaphases are of various shapes due to position of centromere. It is J-shaped when centromere is at :
A)
middle
done
clear
B)
subterminal
done
clear
C)
top
done
clear
D)
near centre
done
clear
View Answer play_arrow
question_answer 130) The term nucleosome was given by Outdet, Oluis and Olius called these particle as nu particles, which histone is absent in nucleosome?
A)
\[{{H}_{1}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[{{H}_{3}}a\]
done
clear
D)
\[{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 131) Nucleosome given beaded appearance to chromosome. They help in packing of DNA in the chromosomes. A nucleosome has :
A)
about 2 turns of DNA
done
clear
B)
8 histone molecules of 4 types (2 mols each of \[{{H}_{2}}\] a,\[{{H}_{2}}\] b, \[{{H}_{3}}\] and\[{{H}_{4}}\])
done
clear
C)
166 nitrogen basepairs
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 132) Salivary glands chromosome were discovered by Balbiani (1881) from salivary glands of larva of :
A)
Chironomous
done
clear
B)
Drosophila
done
clear
C)
Silk worm
done
clear
D)
Lac worm
done
clear
View Answer play_arrow
question_answer 133) In SAT chromosome, SAT (Satellite) is terminal part of chromosome beyond secondary contriction. It contains :
A)
DNA
done
clear
B)
RNA
done
clear
C)
repetitive DNA
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 134) Material exchange through nucleopores is facilitated by :
A)
lamina propria
done
clear
B)
lipid layer
done
clear
C)
nucleoplasmin
done
clear
D)
nucleoles
done
clear
View Answer play_arrow
question_answer 135) Which one of the following enzymes is responsible for the synthesis of DNA from RNA ?
A)
DNA polymerase
done
clear
B)
RNA polymerase
done
clear
C)
Reverse transcriptase
done
clear
D)
DNA ligase
done
clear
View Answer play_arrow
question_answer 136) Where does formation of acetyl Co-A from pyruvic acid take place?
A)
Mitochondria
done
clear
B)
Chloroplast
done
clear
C)
Cytoplasm
done
clear
D)
Golgi body
done
clear
View Answer play_arrow
question_answer 137) Signet ring stage is characteristic of:
A)
pre-erythrocytic schizogony
done
clear
B)
post-erythrocytic schizogony
done
clear
C)
exo-erythrocytic schizogony
done
clear
D)
erythrocytic schizogony
done
clear
View Answer play_arrow
question_answer 138) The phenomenon of regeneration in sponges was observed and explained by :
A)
H.W. Wilson
done
clear
B)
Aristotle
done
clear
C)
Robert Grant
done
clear
D)
Ellis
done
clear
View Answer play_arrow
question_answer 139) Opposite decussate and stipulate leaves is the characteristic feature of members of family :
A)
Rubiaceae
done
clear
B)
Asclepiadaceae
done
clear
C)
Malvaceae
done
clear
D)
Rosaceae
done
clear
View Answer play_arrow
question_answer 140) Persistent calyx forming a cover around the fruit is found in :
A)
Argemone mexicana
done
clear
B)
Ageratiim conyzoides
done
clear
C)
Witionia somnifera
done
clear
D)
Brassica campcstris
done
clear
View Answer play_arrow
question_answer 141) Epigynous and unisexual flowers are found in members of family :
A)
Caryophyllaceae
done
clear
B)
Cumpanulaceae
done
clear
C)
Myrtaceae
done
clear
D)
Cucurbitaceae
done
clear
View Answer play_arrow
question_answer 142) Verticillaster in florescence is the characteristic feature of family :
A)
Euphorbiaceae
done
clear
B)
Moraceae
done
clear
C)
Musaceae
done
clear
D)
Lamiaceae
done
clear
View Answer play_arrow
question_answer 143) Broad spectrum antibiotics are those which :
A)
acts on all bacteria and viruses
done
clear
B)
is effective in very small amounts
done
clear
C)
acts on both pathogen and host
done
clear
D)
acts on a variety of pathogenic
done
clear
View Answer play_arrow
question_answer 144) micro-organisms Find the odd one out:
A)
vaccines - immunology
done
clear
B)
eco degradation - pesticides
done
clear
C)
solar energy converter - pest control
done
clear
D)
recombinant DNA - biotechnology
done
clear
View Answer play_arrow
question_answer 145) The fungus which could bring about hydroxylation required for steroid synthesis is:
A)
Rhizopus stolonifer
done
clear
B)
Aspcrgillus niger
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) Fermentation consist of three steps. The correct sequence of steps is :
A)
inoculation, sterlization and product recovery
done
clear
B)
product recovery, sterlization and inoculation
done
clear
C)
sterlization, inoculation and product recovery
done
clear
D)
sterlization, product recovery and inoculation
done
clear
View Answer play_arrow
question_answer 147) Usually the xylem of fems is made up of:
A)
only tracheids
done
clear
B)
only vessels
done
clear
C)
both tracheids and vessels
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 148) Read the following statement and select the correct answer on the basis of the codes given below the statement:
A)
pteridophytes and gymnosperm are included in archegonide
done
clear
B)
they have archegonid as the female reproductive organ
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 149) In which of the following pairs of genera sporocarps are presents ?
A)
Marsilia and Salvinia
done
clear
B)
Marsilia and Isoetes
done
clear
C)
Isoetes and Salvinia
done
clear
D)
Marsilia and Lycopodium
done
clear
View Answer play_arrow
question_answer 150) In Equisctum stem, the cavity formed by dissolution of protoxylem is known as :
A)
vallicular canal
done
clear
B)
vascular canal
done
clear
C)
carinal canal
done
clear
D)
resin canal
done
clear
View Answer play_arrow
question_answer 151) The scutellum of the grass embryo which is a monocotyledonous is :
A)
photosynthetic organ
done
clear
B)
absorptive organ
done
clear
C)
reserve food storage organ
done
clear
D)
vestigial organ
done
clear
View Answer play_arrow
question_answer 152) During fatique :
A)
blood circulation in muscles stop
done
clear
B)
muscle fails to relax
done
clear
C)
muscles fails to be stimulated
done
clear
D)
motor nerve does not respond to muscles
done
clear
View Answer play_arrow
question_answer 153) Which is true of muscle contraction?
A)
Sarcolemma becomes permeable to \[C{{a}^{2+}}\]ions
done
clear
B)
Sarcolemma becomes permeable to \[\text{N}{{\text{a}}^{\text{+}}}\]ions
done
clear
C)
Sarcolemma becomes non-permeable to\[\text{N}{{\text{a}}^{\text{+}}}\] ions
done
clear
D)
Concentration of \[\text{C}{{\text{a}}^{\text{2+}}}\]ions is reduced in myoblasm
done
clear
View Answer play_arrow
question_answer 154) Stimulus several times greater than threshold stimulus is provided to muscle fibre. It will:
A)
contract with same force
done
clear
B)
contract forcefully
done
clear
C)
contract sightly
done
clear
D)
undergo tetany
done
clear
View Answer play_arrow
question_answer 155) The correct sequence of meninges from inner to outer side is :
A)
arachnoid\[\to \] aurameter\[\to \]piameter
done
clear
B)
arachnoid\[\to \] piameter\[\to \] duramater
done
clear
C)
piamater\[\to \] duramater\[\to \] arachnoid
done
clear
D)
piamater\[\to \] arachnoid\[\to \] duramatar
done
clear
View Answer play_arrow
question_answer 156) Somaesthetic or post central area is responsible for:
A)
initiation of moter impulses for voluntary muscles
done
clear
B)
initiation of moter impulses for involuntary muscles
done
clear
C)
perception of pain, touch and temperature
done
clear
D)
co-ordination of speech
done
clear
View Answer play_arrow
question_answer 157) Vagus nerve is composed mainly of parasympathetic fibres. The preganglionic fibres forms a network in the walls of the organ. This network is known as :
A)
choroid plexus
done
clear
B)
nervous plexus
done
clear
C)
auerbach plexus
done
clear
D)
branchial plexus
done
clear
View Answer play_arrow
question_answer 158) Striated muscles contract because of :
A)
Sliding of myosin rods on actin rods
done
clear
B)
Sliding of actin rods on myosin rods
done
clear
C)
actin rods coming close to each other
done
clear
D)
myosin rods coming close to each other
done
clear
View Answer play_arrow
question_answer 159) Smooth muscles are :
A)
involuntary, spindle shaped, uninucleated, tapering
done
clear
B)
voluntary, multinucleate and cylindrical
done
clear
C)
involuntary, cylindrical, multinucleate
done
clear
D)
voluntary, branched uninuclear
done
clear
View Answer play_arrow
question_answer 160) The term haematocrit means :
A)
the percentage of blood that is red blood cells
done
clear
B)
the ratio of blood volume tb extracellular spaced
done
clear
C)
the percentage of new blood cells formed every 120 days
done
clear
D)
the percentage of blood that is white blood cells
done
clear
View Answer play_arrow
question_answer 161) What is the main difference in human and frog RBC?
A)
Human RBC are non-nucleated
done
clear
B)
Haemoglobin is found only in human RBC
done
clear
C)
Human RBC have nucleus
done
clear
D)
Human RBC are multinucleated
done
clear
View Answer play_arrow
question_answer 162) Prothrombin is found in :
A)
intestine and helps in cellulose digestion
done
clear
B)
lever and helps in production of bile
done
clear
C)
blood and gives red colour
done
clear
D)
blood and helps in blood clotting
done
clear
View Answer play_arrow
question_answer 163) Which type of WBCs are most abundant in blood of rabbit and other vertibrates ?
A)
Acidophils
done
clear
B)
Basophils
done
clear
C)
Lymphocytes
done
clear
D)
Neutrophils
done
clear
View Answer play_arrow
question_answer 164) Blood clotting in a test tube can be prevented by adding a little of :
A)
sodium oxalate
done
clear
B)
sodium chloride
done
clear
C)
sodium hydroxide
done
clear
D)
ammonium chloride
done
clear
View Answer play_arrow
question_answer 165) Oval, biconvex and nucleated RBCs are found in :
A)
camel
done
clear
B)
rabbit
done
clear
C)
man
done
clear
D)
frog
done
clear
View Answer play_arrow
question_answer 166) Which of the following is an anticoagulant and checks blood coagulation in blood vessels ?
A)
Prothrombin
done
clear
B)
Globulin
done
clear
C)
Thromboplastin
done
clear
D)
Heparin
done
clear
View Answer play_arrow
question_answer 167) In normal healthy female, the number of RBC/mm3 of blood is :
A)
6.5-7.0 million
done
clear
B)
5.5-6.0 million
done
clear
C)
4.5-5.0 million
done
clear
D)
3.5-4.0 million
done
clear
View Answer play_arrow
question_answer 168) The tissue which forms the basic structure of lymphoid organs, liver etc., is :
A)
lymphoid tissue
done
clear
B)
reticular tissue
done
clear
C)
elaster tissue
done
clear
D)
areolar tissue
done
clear
View Answer play_arrow
question_answer 169) Development of embryo from a cell of embryo sac other than egg is an example of :
A)
apospory
done
clear
B)
apogamy
done
clear
C)
adventive embryogemy
done
clear
D)
parthenogenesis
done
clear
View Answer play_arrow
question_answer 170) Suppose evolution on earth has occurred in such a way that there are 96 amino acids instead of 20. DNAhas 12 different types of bases and DNA synthesis occur in the same way as today. The minimum number of bases per DNA codon would be :
A)
12
done
clear
B)
8
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 171) Haemolytic jaundice is due to a dominant gene but only 10% of the people develops this disease. A heterozygous male marries a homozygous normal woman. What proportion of the children in population would be expected to have this disorder?
A)
\[1/5\]
done
clear
B)
\[1/10\]
done
clear
C)
\[1/20\]
done
clear
D)
\[1/2\]
done
clear
View Answer play_arrow
question_answer 172) Which of the following should be avoided in bilogical marriages ?
A)
\[{{A}^{+}}\] boy and \[{{A}^{+}}\] girl
done
clear
B)
\[{{A}^{+}}\] boy and \[{{A}^{-}}\]girl
done
clear
C)
\[{{O}^{-}}\] boy and \[{{O}^{+}}\] girl
done
clear
D)
\[{{O}^{-}}\] boy and \[{{O}^{+}}\] girl
done
clear
View Answer play_arrow
question_answer 173) After examining the blood group of husband and wife, the doctor advised them not to have more than one child. The blood groups of the couple are likely to be :
A)
male \[R{{h}^{-}}\] and female \[R{{h}^{+}}\]
done
clear
B)
femal \[R{{h}^{-}}\] and male \[R{{h}^{+}}\]
done
clear
C)
male \[R{{h}^{+}}\] and female \[R{{h}^{+}}\]
done
clear
D)
male \[R{{h}^{-}}\] and female \[R{{h}^{-}}\]
done
clear
View Answer play_arrow
question_answer 174) The number of ATP molecules produced by electron transport system from Krebs cycle intermediates in a single turn is :
A)
11
done
clear
B)
14
done
clear
C)
12
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 175) The enzymes called marker enzymes in lysosome is :
A)
acid phosphatase
done
clear
B)
hydrolase
done
clear
C)
hyaluronidase
done
clear
D)
lysozyme
done
clear
View Answer play_arrow
question_answer 176) Centrioles (microcentrosome or cell centre) is:
A)
microtubuler and membraneles
done
clear
B)
absent in Amoeba, red algae, blue-green algae, conifers and angiosperm and made up of a peripheral implet microtubular
done
clear
C)
basically locomotary and their role in spindle formation is secondary
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 177) Modules which are present in leguminous plants are meant for fertilizers and are found in/on:
A)
certain leguminous plants
done
clear
B)
leschynomene
done
clear
C)
Scsbania rostcrata
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 178) A mutation in bacterial results in non-formation of mesosomes. The expected results will be :
A)
only cell division will occur
done
clear
B)
only replication of DNA will occur
done
clear
C)
only karyokinesis will occur
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 179) Agriculturists have reported about 40-50% higher yields of rice by applying :
A)
Azolla pinnata
done
clear
B)
cyanophycean members
done
clear
C)
mycorrhizae
done
clear
D)
thorn forest
done
clear
View Answer play_arrow
question_answer 180) Bacterial toxins when excreted into the surrounding medium are known as :
A)
toxins
done
clear
B)
endotoxins
done
clear
C)
exotoxins
done
clear
D)
both [b] and [c]
done
clear
View Answer play_arrow
question_answer 181) Antibacterial and antifungal antibiotic called citrinin is obtained from :
A)
Aspergillus flavus
done
clear
B)
Sfreptomyces erythraeus
done
clear
C)
Ustilaga zeae
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 182) Light coloured peppered moth/Biston betularia gets changed to its darker carbonaria variety due to :
A)
translocation of block of genes in response to heavy carbons
done
clear
B)
deletion of gene segment due to industrial pollution
done
clear
C)
mutation of single Mendelian gene for survival in smoke industrial environment
done
clear
D)
industrial carbon deposited on wings
done
clear
View Answer play_arrow
question_answer 183) Organisms have a tendency towards increase in size during their evolution; is :
A)
Hulls Rule
done
clear
B)
Copes Rule
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 184) Modern synthetic theory recognises five basic types of processes for evolution wliich of the following two processes gluds the population into adaptive channels?
A)
Gene mutation and natural selection
done
clear
B)
Genetic recombination and reproductive isolation
done
clear
C)
Natural selection and reproductive isolation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 185) Where there is sustained directional change in the average characters of a population due to adaptation to a shifting environment is called:
A)
quantum evolution
done
clear
B)
phyletic evolution
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 186) When no new population are formed a result of miner changes in the gene pool of a population from one generation to next, the process is known as:
A)
convergent evolution
done
clear
B)
divergent evolution
done
clear
C)
sequential evolution
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 187) The major role in formation of new species from old species is played by :
A)
autoploidy
done
clear
B)
allopolyploidy
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 188) The type of speciation which results due to chromosomal aberration (like inversion and translocation) change in chromosomes number (polyploidy, autopolyploidy etc.) is known as :
A)
phyletic speciation
done
clear
B)
quantum speciation
done
clear
C)
gradual speciation
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 189) Relationship between hermit crab and sea anaemone shows which type of association ?
A)
Commensalism
done
clear
B)
Symbiosis
done
clear
C)
Mutualism
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 190) Which of the following can convert \[N{{H}_{3}}/N{{O}_{3}}\] into amino acid ?
A)
Reducers
done
clear
B)
Osmotrophs
done
clear
C)
Transducers
done
clear
D)
Detrivores
done
clear
View Answer play_arrow
question_answer 191) The natural cycling of carbon between organism and their environment is directly accomplished through :
A)
radiations of solar energy
done
clear
B)
photosynthesis and respiration
done
clear
C)
nutrition and excretion
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 192) Acid rain in some industrial cities are due to :
A)
excessive release of \[C{{O}_{2}}\] by burning of fossil fuels
done
clear
B)
excessive concentration of \[S{{O}_{2}}\] and \[N{{O}_{2}}\]in air by deforestation
done
clear
C)
excessive release of \[S{{O}_{2}}\]by industries and coal gas
done
clear
D)
deforestation, population explosion and release of \[C{{O}_{2}}\]by automobile exhausts
done
clear
View Answer play_arrow
question_answer 193) According to our national forest policy, forests are divided into :
A)
2 categories
done
clear
B)
8 categories
done
clear
C)
5 categories
done
clear
D)
3 categories
done
clear
View Answer play_arrow
question_answer 194) Which of the following is the first fossil fuel exploited for commercial energy ?
A)
Natural gas
done
clear
B)
Coal
done
clear
C)
Oil shale
done
clear
D)
Petroleum
done
clear
View Answer play_arrow
question_answer 195) The biome, which is characterised by broad-leaved vegetation, fire resistant resinous plants and drough evading plant is known as :
A)
sleppes
done
clear
B)
savanna
done
clear
C)
deciduous forests
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 196) The plants species which is on the verge of extinction due to over exploitation is/are :
A)
gloriosa
done
clear
B)
podophylum
done
clear
C)
ceritella
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 197) The addition of any substance to water which leads to change in its physical and chemical characteristic and which infere is its legitimate purpose is defined as water pollution. It results in :
A)
decrease turibidity
done
clear
B)
increase oxygenation
done
clear
C)
increase photosynthesis
done
clear
D)
increase turbidity deoxygenating
done
clear
View Answer play_arrow
question_answer 198) According ro current views the role of boron in plants is in the biosynthesis of :
A)
sucrose and amylopectin
done
clear
B)
cellulose and starch
done
clear
C)
fats and oils
done
clear
D)
pectin and DNA
done
clear
View Answer play_arrow
question_answer 199) A nutrient element essential for the formation of micro-tubules of the mitotic spindle apparatus during cell division is :
A)
phosphorus
done
clear
B)
sulphur
done
clear
C)
calcium
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 200) The first electron acceptor in photosystem-I of cyclic photophosphorylation is :
A)
cytochrome
done
clear
B)
plastocyanin
done
clear
C)
ferrodoxin
done
clear
D)
plastoquinone
done
clear
View Answer play_arrow