A) hyperconjugative effect of Me-group in\[MeN{{H}_{2}}\]
B) resonance effect of phenyl group in aniline
C) lower molecular weight of methyl amine as compared to that of aniline
D) resonance effect of\[-N{{H}_{2}}\] group in \[MeN{{H}_{2}}\]
Correct Answer: B
Solution :
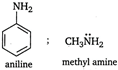 The lone pair of electrons, present in N-atom in aniline involve in ring resonance. So it is less basic than methyl amine.
The lone pair of electrons, present in N-atom in aniline involve in ring resonance. So it is less basic than methyl amine.
You need to login to perform this action.
You will be redirected in
3 sec
