A) 1, 2-dinitrobenzene
B) 1, 3-dinitrobenzene
C) 1.4-dinitrobenzene
D) 1, 2, 4-trinitrobenzene
Correct Answer: B
Solution :
\[N{{O}_{2}}\]group being electron withdrawing reduces electron density at output positions. Hence, now the mefa-position becomes electron rich on which the electrophile (nitronium ion) attacks during nitration. \[HN{{O}_{3}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}{{H}_{2}}NO_{3}^{+}+HSO_{4}^{-}\] \[{{H}_{2}}O+\underset{\text{electrophile}}{\mathop{NO_{2}^{+}}}\,\]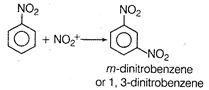
You need to login to perform this action.
You will be redirected in
3 sec
