A) \[\frac{1}{2}({{\rho }_{1}}-{{\rho }_{2}})({{V}_{1}}-{{V}_{2}})\]
B) \[\frac{1}{2}({{\rho }_{1}}+{{\rho }_{2}})({{V}_{1}}-{{V}_{2}})\]
C) \[\frac{1}{2}\left( {{p}_{1}}+\frac{a}{V_{1}^{2}}-{{p}_{2}}-\frac{a}{V_{2}^{2}} \right)({{V}_{1}}-{{V}_{2}})\]
D) \[\frac{1}{2}\left( {{p}_{1}}+\frac{a}{V_{1}^{2}}+{{p}_{2}}+\frac{a}{V_{2}^{2}} \right)({{V}_{1}}-{{V}_{2}})\]
Correct Answer: A
Solution :
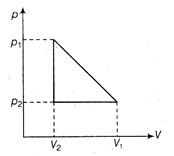
For the cyclic process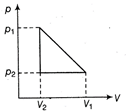 Heat absorbed = Work done \[=Area=\frac{1}{2}(\Delta p)\times \Delta V\] \[=\frac{1}{2}({{p}_{1}}-{{p}_{2}})\times ({{V}_{1}}-{{V}_{2}})\] \[=\frac{1}{2}({{p}_{1}}-{{p}_{2}})({{V}_{1}}-{{V}_{2}})\]
Heat absorbed = Work done \[=Area=\frac{1}{2}(\Delta p)\times \Delta V\] \[=\frac{1}{2}({{p}_{1}}-{{p}_{2}})\times ({{V}_{1}}-{{V}_{2}})\] \[=\frac{1}{2}({{p}_{1}}-{{p}_{2}})({{V}_{1}}-{{V}_{2}})\]
You need to login to perform this action.
You will be redirected in
3 sec
