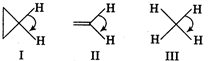
A) I > II > III
B) II > I > III
C) III > II > I
D) I > III > II
Correct Answer: B
Solution :
H-C-H bond angle is about \[120{}^\circ \], when C is bonded through a double bond but in case of \[\text{C}{{\text{H}}_{\text{4}}}\text{,}\]the H-C-H bond angle is only \[\text{10}{{\text{9}}^{o}}\,28.\] Cyclic compounds have a H-C-H bond angle greater than \[\text{10}{{\text{9}}^{o}}\,28\] due to hindrance. Thus, the correct order of H- C-H bond angle is \[\text{II}\,\text{}\,\text{I}\,\text{}\,\text{II}\]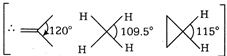
You need to login to perform this action.
You will be redirected in
3 sec
