A) \[\underset{CHO}{\mathop{\underset{|}{\mathop{C{{H}_{2}}OH}}\,}}\,\]
B) \[\underset{C{{O}_{2}}H}{\overset{CHO}{\mathop{|}}}\,\]
C) \[\underset{C{{O}_{2}}H}{\overset{C{{H}_{2}}OH}{\mathop{|}}}\,\]
D) \[\underset{C{{O}_{2}}H}{\overset{C{{O}_{2}}H}{\mathop{|}}}\,\]
Correct Answer: C
Solution :
Aldehydes lacking \[\alpha -H\] atom, undergo Cannizzaro reaction if treated with concentrated alkali (NaOH), i.e., one mole of the aldehyde is reduced to alcohol and other mole is oxidised to salt of acid. Thus, the given aldehyde (glyoxal) undergoes intramolecular Cannizzaro reaction, i.e., its half part is oxidised and half is reduced.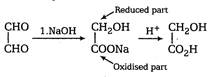
You need to login to perform this action.
You will be redirected in
3 sec
