A) \[A{{g}_{2}}{{S}_{2}}{{O}_{3}},N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}S\]
B) \[A{{g}_{2}}S{{O}_{4}},Na[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}{{S}_{2}}\]
C) \[A{{g}_{2}}{{S}_{2}}{{O}_{3}},N{{a}_{5}}[Ag{{({{S}_{2}}{{O}_{3}})}_{3}}],AgS\]
D) \[A{{g}_{2}}S{{O}_{3}},N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}],A{{g}_{2}}O\]
Correct Answer: A
Solution :
The sequence of reactions are \[\underset{\begin{smallmatrix} Silver \\ nitrate \end{smallmatrix}}{\mathop{2AgN{{O}_{3}}}}\,+\underset{\begin{smallmatrix} Sodium \\ thiosulphate \end{smallmatrix}}{\mathop{N{{a}_{2}}{{S}_{2}}{{O}_{3}}}}\,\xrightarrow{{}}\underset{\begin{smallmatrix} (x) \\ (White\,ppt.) \end{smallmatrix}}{\mathop{A{{g}_{2}}{{S}_{2}}{{O}_{3}}}}\,+2NaN{{O}_{3}}\]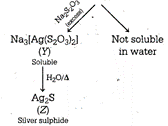 Hence, \[X=A{{g}_{2}}{{S}_{2}}{{O}_{3}}\] \[Y=N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]Z=A{{g}_{2}}S\]
Hence, \[X=A{{g}_{2}}{{S}_{2}}{{O}_{3}}\] \[Y=N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]Z=A{{g}_{2}}S\]
You need to login to perform this action.
You will be redirected in
3 sec
