question_answer 1) Acceleration due to gravity is g on the surface of the earth. Then the value of the acceleration due to gravity at a height of 32 km above earths surface is (Assume radius of earth to be 6400 km)
A)
0.99 g
done
clear
B)
0.8 g
done
clear
C)
1.01 g
done
clear
D)
0.9 g
done
clear
View Answer play_arrow
question_answer 2) A piece of ice is floating in a jar containing water. When the ice melts, then the level of water......
A)
rises
done
clear
B)
falls
done
clear
C)
remains unchanged
done
clear
D)
rises or falls
done
clear
View Answer play_arrow
question_answer 3) A wire of length L and area of cross-section A is stretched through a distance \[x\]metre by applying a force F along length, then the work done in this process is (Y is Youngs modulus of the material)
A)
\[\frac{1}{2}(A.L)\left( \frac{Yx}{L} \right)\left( \frac{x}{L} \right)\]
done
clear
B)
\[(A.L)(YL)\left( \frac{x}{L} \right)\]
done
clear
C)
\[2(A.L)(YL)\left( \frac{x}{L} \right)\]
done
clear
D)
\[3(A.L)(YL)\left( \frac{x}{L} \right)\]
done
clear
View Answer play_arrow
question_answer 4) An ice box made of Styrofoam (Thermal conductivity \[=0.01\,J{{m}^{-1}}{{s}^{-1}}{{K}^{-1}}\] is used to keep liquids cool. It has a total wall area Including lid of \[\text{0}\text{.8}\,{{\text{m}}^{\text{2}}}\]and wall thickness of 2.0 cm. A bottle of water is placed in the box and filled with ice. If the outside temperature is \[\text{3}{{\text{0}}^{\text{o}}}\text{C}\] the rate of flow of heat into the box is (in\[\text{J}-{{s}^{-1}}\])
A)
16
done
clear
B)
14
done
clear
C)
12
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 5) The KE and PE of a particle executing SHM of amplitude a will be equal when displacement is
A)
\[\frac{a}{2}\]
done
clear
B)
\[a\sqrt{2}\]
done
clear
C)
\[2a\]
done
clear
D)
\[\frac{a}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 6) The equation \[y=A\sin 2\pi \left( \frac{t}{T}-\frac{x}{\lambda } \right),\] where the symbols carry the usual meaning and A, T and \[\lambda \] are positive, represents a wave of
A)
amplitude 2 A
done
clear
B)
period \[T/\lambda \]
done
clear
C)
speed\[x\lambda \]
done
clear
D)
speed\[\left( \frac{\lambda }{T} \right)\]
done
clear
View Answer play_arrow
question_answer 7) A pipe open at both the ends produces a note of fundamental frequency \[{{f}_{1}}.\] When the pipe is 3 kept with \[\frac{3}{4}\text{th}\] of its length in water, it produces 4 a note of fundamental frequency \[{{f}_{2}}.\] The ratio of\[\frac{{{f}_{1}}}{{{f}_{2}}}\] is
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{3}{4}\]
done
clear
C)
2
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 8) The potential at a point P which is forming a comer of a square of side 93 mm with charges, \[{{Q}_{1}}=33\,nC,\,{{Q}_{2}}=-\,51nC,\,{{Q}_{3}}=47\,nC\] located at the other three comers is nearly
A)
\[16\,kV\]
done
clear
B)
\[4\,kV\]
done
clear
C)
\[400\,V\]
done
clear
D)
\[160\,V\]
done
clear
View Answer play_arrow
question_answer 9)
A)
8:1
done
clear
B)
1 : 8
done
clear
C)
2:1
done
clear
D)
1:2
done
clear
View Answer play_arrow
question_answer 10) The electric potential due to a small electric dipole at a large distance r from the centre of the dipole is proportional to
A)
r
done
clear
B)
\[\frac{1}{r}\]
done
clear
C)
\[\frac{1}{{{r}^{5}}}\]
done
clear
D)
\[\frac{1}{{{r}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 11)
In the given circuit the equivalent resistance between the points A and B in ohms is ........
A)
9
done
clear
B)
11.6
done
clear
C)
14.5
done
clear
D)
21.2
done
clear
View Answer play_arrow
question_answer 12) An electric water kettle rated 2.1 kW is filled with 1.5 kg of water at \[\text{20}{{\,}^{\text{o}}}\text{C}\text{.}\] How many seconds does it take to reach the boiling point of water? Assume that there are no heat losses from the kettle. Specific heat capacity of water is \[\text{Jk}{{\text{g}}^{-1}}{{\text{K}}^{-1}}\]
A)
60
done
clear
B)
120
done
clear
C)
240
done
clear
D)
480
done
clear
View Answer play_arrow
question_answer 13)
In the electric circuit shown each cell has an emf of 2 V and internal resistance of \[1\Omega .\]The external resistance is \[2\Omega .\] The value of the current \[\text{I}\] is (in amperes)
A)
2
done
clear
B)
1.25
done
clear
C)
0.4
done
clear
D)
1.2
done
clear
View Answer play_arrow
question_answer 14) An immersion heater with electrical resistance \[\text{7}\Omega \] is immersed in 0.1 kg of water at \[\text{20}{{\,}^{\text{o}}}\text{C}\] for 3 min. If the flow of current is 4 A, what is the final temperature of the water in ideal conditions? (Specific heat capacity of water\[\text{=}\,\text{4}\text{.2}\times {{10}^{3}}\,JK{{g}^{-1}}{{K}^{-1}}\])
A)
\[\text{28}{{\,}^{\text{o}}}\text{C}\]
done
clear
B)
\[\text{48}{{\,}^{\text{o}}}\text{C}\]
done
clear
C)
\[\text{52}{{\,}^{\text{o}}}\text{C}\]
done
clear
D)
\[68{{\,}^{\text{o}}}\text{C}\]
done
clear
View Answer play_arrow
question_answer 15) Heat produced (cals) in a resistance R When a current \[I\] amperes flows through it fort seconds is given by the expression
A)
\[\frac{{{I}^{2}}Rt}{4.2}\]
done
clear
B)
\[\frac{I{{R}^{2}}t}{4.2}\]
done
clear
C)
\[\frac{4.2\,IR}{{{t}^{2}}}\]
done
clear
D)
\[\frac{IR{{t}^{2}}}{4.2}\]
done
clear
View Answer play_arrow
question_answer 16) Which of the following graphs represent variation of magnetic field B with distance r for a straight long wire carrying current?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 17) If a coil of 40 turns and area \[\text{40}\,\text{c}{{\text{m}}^{\text{2}}}\] is suddenly removed from a magnetic field, it is observed that a charge of \[2.0\times {{10}^{-4}}C\] flows into the coil. If the resistance of the coil is \[80\Omega ,\] the magnetic flux density in \[\text{Wb/}{{\text{m}}^{\text{2}}}\] is .......
A)
0.5
done
clear
B)
1.0
done
clear
C)
1.5
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 18) The maximum value of AC voltage in a circuit is 707 V. Its rms value is
A)
70.7V
done
clear
B)
100V
done
clear
C)
500V
done
clear
D)
707V
done
clear
View Answer play_arrow
question_answer 19) The magnetic flux linked with the coil varies with time as \[\text{o }\!\!|\!\!\text{ =3}{{\text{t}}^{2}}+4t+9.\] The magnitude of the induced emf at 2 s is
A)
9V
done
clear
B)
16V
done
clear
C)
3V
done
clear
D)
4V
done
clear
View Answer play_arrow
question_answer 20) Band spectrum is also called
A)
molecular spectrum
done
clear
B)
atomic spectrum
done
clear
C)
flash spectrum
done
clear
D)
line absorption spectrum
done
clear
View Answer play_arrow
question_answer 21) A fish at a depth of 12 cm in water is viewed by an observer on the bank of a lake. To what height the image of the fish is raised? (Refractive index of lake water = 4/3)
A)
9 cm
done
clear
B)
12cm
done
clear
C)
3.8 cm
done
clear
D)
3 cm
done
clear
View Answer play_arrow
question_answer 22) A ray of light is incident at \[\text{5}{{\text{0}}^{\text{o}}}\] on the middle of one of the two mirrors arranged at an angle of \[\text{6}{{\text{0}}^{\text{o}}}\] between them. The ray then touches the second mirror, gets reflected back to the first mirror, making an angle of incidence .....
A)
\[\text{5}{{\text{0}}^{\text{o}}}\]
done
clear
B)
\[\text{6}{{\text{0}}^{\text{o}}}\]
done
clear
C)
\[\text{7}{{\text{0}}^{\text{o}}}\]
done
clear
D)
\[\text{8}{{\text{0}}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 23) In a compound microscope, the intermediate image is
A)
virtual, erect and magnified
done
clear
B)
real, erect and magnified
done
clear
C)
real, inverted and magnified
done
clear
D)
virtual, erect and reduced
done
clear
View Answer play_arrow
question_answer 24) A ray of light passes through an equilateral prism such that an angle of incidence is equal to the angle of emergence and the latter is equal to \[\frac{3}{4}\text{th}\] the angle of prism. The angle of deviation is
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{39}^{o}}\]
done
clear
C)
\[\text{2}{{\text{0}}^{\text{o}}}\]
done
clear
D)
\[\text{3}{{\text{0}}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 25) An electron enters uniform electric field maintained by parallel plates and of value \[\text{EV}{{\text{m}}^{-1}}\] with a velocity \[\text{v}\,\text{m}{{\text{s}}^{-1}},\] the plates are separated by a distance d metre, then acceleration of the electron in the field is
A)
\[\frac{Ee}{m}\]
done
clear
B)
\[\frac{-Ee}{m}\]
done
clear
C)
\[\frac{Ed}{m}\]
done
clear
D)
\[Ee\frac{d}{m}\]
done
clear
View Answer play_arrow
question_answer 26) \[{{\text{C}}^{\text{14}}}\] has half-life 5700 yr. At the end of 11400 yr, the actual amount left is
A)
0.5 of original amount
done
clear
B)
0.25 of original amount
done
clear
C)
0.125 of original amount
done
clear
D)
0.0625 of original amount
done
clear
View Answer play_arrow
question_answer 27) The ionization potential of hydrogen is 13.6 V. The energy required to remove an electron from the second orbit of hydrogen is
A)
3.4 eV
done
clear
B)
6.8 eV
done
clear
C)
13.6 eV
done
clear
D)
1.51 eV
done
clear
View Answer play_arrow
question_answer 28) The depletion layer of a\[p-n\] junction
A)
is of constant width irrespective of the bias
done
clear
B)
acts like an insulating zon& under reverse bias
done
clear
C)
has a width that increases with an increase in forward bias
done
clear
D)
is depleted of ions
done
clear
View Answer play_arrow
question_answer 29) Li nucleus has three protons and four neutrons. Mass of lithium nucleus is 7.016005 amu. Mass of proton is 1.007277 amu and mass of neutronis 1.008665 amu. Mass defect for lithium nucleus in amu is
A)
0.04048 amu
done
clear
B)
0.04050 amu
done
clear
C)
0.04052 amu
done
clear
D)
0.04055 amu
done
clear
View Answer play_arrow
question_answer 30) The dimensions of kinetic energy is
A)
\[[{{M}^{2}}{{L}^{2}}T]\]
done
clear
B)
\[[M{{L}^{2}}T]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 31)
If the figure below represents a parabola, identify the physical quantities representing Y and X for constant acceleration
A)
X= time, Y= velocity
done
clear
B)
X = velocity, Y = time
done
clear
C)
X = time, Y = displacement
done
clear
D)
X = time, Y =acceleration
done
clear
View Answer play_arrow
question_answer 32) A ball which is at rest is dropped from a height h metre. As it bounces off the floor its speed is 80% of what it was just before touching the ground. The ball will then rise to nearly a height
A)
0.94 h
done
clear
B)
0.80 h
done
clear
C)
0.75 h
done
clear
D)
0.64 h
done
clear
View Answer play_arrow
question_answer 33) Two bodies of different masses are dropped from heights of 16 m and 25 m respectively. The ratio of the time taken by them to reach the ground is
A)
\[\frac{25}{16}\]
done
clear
B)
\[\frac{5}{4}\]
done
clear
C)
\[\frac{4}{5}\]
done
clear
D)
\[\frac{16}{25}\]
done
clear
View Answer play_arrow
question_answer 34) The recoil velocity of a 4.0 kg rifle that shoots a 0.050 kg bullet at a speed of \[280\,\text{m}{{\text{s}}^{-1}}\] is
A)
\[+\,3.5\,m{{s}^{-1}}\]
done
clear
B)
\[-\,3.5\,m{{s}^{-1}}\]
done
clear
C)
\[-\sqrt{(3.5)}m{{s}^{-1}}\]
done
clear
D)
\[+\sqrt{(3.5)}\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 35) A man of height h walks in a straight path towards a latnp post of height H with uniform velocity u. Then the velocity of the edge of the shadow on the ground will be
A)
\[\frac{hu}{(H-h)}\]
done
clear
B)
\[\frac{Hu}{(H+h)}\]
done
clear
C)
\[\frac{(H-h)}{Hu}\]
done
clear
D)
\[\frac{(H+h)}{Hu}\]
done
clear
View Answer play_arrow
question_answer 36) A train of 150 m length is going towards north direction at a speed of \[10\,\text{m}{{\text{s}}^{-1}}.\] A parrot flies at a speed of \[\text{5}\,\text{m}{{\text{s}}^{-1}}\] towards south direction parallel to the railway track. The time taken by the parrot to cross the train is equal to
A)
12s
done
clear
B)
8 s
done
clear
C)
15s
done
clear
D)
10 s
done
clear
View Answer play_arrow
question_answer 37) In the motion of a rocket, physical quantity which is conserved is
A)
angular momentum
done
clear
B)
linear momentum
done
clear
C)
force
done
clear
D)
work
done
clear
View Answer play_arrow
question_answer 38) An aeroplane is flying horizontally with a velocity of 600 km/h and at a height of 1960 m. When it is vertically above a point A on the ground a bomb is released from it. The bomb strikes the ground at point B. The distance AB is
A)
1200m
done
clear
B)
0.33km
done
clear
C)
333.3km
done
clear
D)
3.33km
done
clear
View Answer play_arrow
question_answer 39) A 10 kg object collides with stationary 5 kg object and after collision they stick together and move forward with velocity \[4\,\text{m}{{\text{s}}^{-1}}.\]What is the velocity with which the 10 kg object hit the second one?
A)
\[4\,m{{s}^{-1}}\]
done
clear
B)
\[6\,m{{s}^{-1}}\]
done
clear
C)
\[10\,m{{s}^{-1}}\]
done
clear
D)
\[12\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 40) When a force is applied on a moving body, its motion is retarded. Then the work done is
A)
positive
done
clear
B)
negative
done
clear
C)
zero
done
clear
D)
positive and negative
done
clear
View Answer play_arrow
question_answer 41) Physical independence of force is a consequence of
A)
third law of motion
done
clear
B)
second law of motion
done
clear
C)
first law of motion
done
clear
D)
all of these laws
done
clear
View Answer play_arrow
question_answer 42) The kinetic energy of a body becomes four times its initial value. The new momentum will be ...............
A)
same as the initial value
done
clear
B)
twice the initial value
done
clear
C)
thrice the initial value
done
clear
D)
half of its initial value
done
clear
View Answer play_arrow
question_answer 43) An ice cart of mass 60 kg rests on a horizontal snow patch with coefficient of static friction \[\frac{1}{3}.\] Assuming that there is no vertical acceleration, find the magnitude of the maximum horizontal force required to move the ice cart. \[(g=9.8\,m{{s}^{-2}})\]
A)
100 N
done
clear
B)
110 N
done
clear
C)
209 N
done
clear
D)
196 N
done
clear
View Answer play_arrow
question_answer 44) Moment of a couple is called
A)
impulse
done
clear
B)
couple
done
clear
C)
torque
done
clear
D)
angular momentum
done
clear
View Answer play_arrow
question_answer 45) A cyclist is moving in a circular track of radius 80 m with a velocity v = 36 km/h. He has to lean from the vertical approximately through an angle (Take \[g=10\,m{{s}^{-2}}\])
A)
\[{{\tan }^{-1}}(4)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{1}{3} \right)\]
done
clear
C)
\[{{\tan }^{-1}}\left( \frac{1}{4} \right)\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{1}{8} \right)\]
done
clear
View Answer play_arrow
question_answer 46) An athlete throws a discus from rest to a final angular velocity of 15 rad/s in 0.270 s before releasing it. During acceleration, discus moves a circular arc of radius 0.810 m. Acceleration of discus before it is released is..... . \[m{{s}^{-2}}.\]
A)
45
done
clear
B)
182
done
clear
C)
187
done
clear
D)
192
done
clear
View Answer play_arrow
question_answer 47) A motor is rotating at a constant angular velocity of 600 rpm. The angular displacement per second is
A)
\[\frac{3}{50\pi }\,rad\]
done
clear
B)
\[\frac{3\pi }{50}\,rad\]
done
clear
C)
\[\frac{25\,\pi }{3}\,rad\]
done
clear
D)
\[\frac{50\,\pi }{3}\,rad\]
done
clear
View Answer play_arrow
question_answer 48) If the mass of moon is\[\frac{1}{90}\] of earths m ass, its radius is\[\frac{1}{3}\]of earths radius and if g is acceleration due to gravity on earth, then the acceleration due to gravity on moon is ......
A)
\[\frac{g}{3}\]
done
clear
B)
\[\frac{g}{90}\]
done
clear
C)
\[\frac{g}{10}\]
done
clear
D)
\[\frac{g}{9}\]
done
clear
View Answer play_arrow
question_answer 49) A red coloured object illuminated by mercury vapour lamp, when seen through a green filter, will appear
A)
red
done
clear
B)
blue
done
clear
C)
violet
done
clear
D)
black
done
clear
View Answer play_arrow
question_answer 50) Time taken by sunlight to pass through a window of thickness 4 mm whose refractive index is\[\frac{3}{2}\]is
A)
\[2\times {{10}^{-4}}s\]
done
clear
B)
\[2\times {{10}^{4}}s\]
done
clear
C)
\[2\times {{10}^{-11}}s\]
done
clear
D)
\[2\times {{10}^{11}}s\]
done
clear
View Answer play_arrow
question_answer 51) Two thin lenses of focal length 20 cm and 25 cm are in contact. The effective power of the combination is
A)
4.5 D
done
clear
B)
18 D
done
clear
C)
45 D
done
clear
D)
9 D
done
clear
View Answer play_arrow
question_answer 52) The magnification of the image when an object is placed at a distance \[x\]from the principal focus of a mirror of focal length\[f\] is
A)
\[\frac{x}{f}\]
done
clear
B)
\[1+\frac{f}{x}\]
done
clear
C)
\[\frac{f}{x}\]
done
clear
D)
\[1-\frac{f}{x}\]
done
clear
View Answer play_arrow
question_answer 53) In the Youngs double slit experiment, the central maxima is observed to be \[{{\text{I}}_{\text{0}}}\text{.}\]If one of the slits is covered, then the intensity at the central maxima will become
A)
\[\frac{{{I}_{0}}}{2}\]
done
clear
B)
\[\frac{{{I}_{0}}}{\sqrt{2}}\]
done
clear
C)
\[\frac{{{I}_{0}}}{4}\]
done
clear
D)
\[{{I}_{0}}\]
done
clear
View Answer play_arrow
question_answer 54) The ratio of the de-Broglie wavelength of an \[\alpha -\]particle and a proton of same kinetic energy is
A)
1:2
done
clear
B)
1:1
done
clear
C)
\[1:\sqrt{2}\]
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 55) Which of the following is not conserved in nuclear reaction?
A)
Total energy
done
clear
B)
Mass number
done
clear
C)
Charge number
done
clear
D)
Number of fundamental particles
done
clear
View Answer play_arrow
question_answer 56) The number of a-particles and \[\beta -\]particles respectively emitted in the reaction\[{{\,}_{88}}{{A}^{196}}\to {{\,}_{78}}{{B}^{164}}\] are
A)
8 and 8
done
clear
B)
8 and 6
done
clear
C)
6 and 8
done
clear
D)
6 and 6
done
clear
View Answer play_arrow
question_answer 57) The counting rate observed from a radioactive source at t = 0 was 1600 count/s and at t = 8 s it was 100 counts/s. The counting rate observed as counts per second at t = 6 s, will be
A)
400
done
clear
B)
300
done
clear
C)
250
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 58) If \[{{n}_{1}}\]and \[{{n}_{2}}\]are the refractive indices of the core and the cladding respectively of an optic fibre, then
A)
\[{{n}_{1}}={{n}_{2}}\]
done
clear
B)
\[{{n}_{1}}<{{n}_{2}}\]
done
clear
C)
\[{{n}_{2}}<{{n}_{1}}\]
done
clear
D)
\[{{n}_{2}}=2{{n}_{1}}\]
done
clear
View Answer play_arrow
question_answer 59) The dimensional formula of magnetic flux is
A)
\[[ML{{T}^{-2}}{{A}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-1}}{{A}^{-1}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-1}}{{A}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}{{A}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 60) A physical quantity A is related to four observables a, b, c and d as follows\[A=\frac{{{a}^{2}}{{b}^{3}}}{c\sqrt{d}}\] The percentage errors of measurement in a, b, c and d are 1%, 3%, 2% and 2% respectively. What is the percentage error in the quantity A?
A)
12%
done
clear
B)
7%
done
clear
C)
5%
done
clear
D)
14%
done
clear
View Answer play_arrow
question_answer 61) A body starting from rest moves with constant acceleration. The ratio of distance covered by the body during the 5th second to that covered in 5 s is
A)
\[\frac{9}{25}\]
done
clear
B)
\[\frac{3}{5}\]
done
clear
C)
\[\frac{25}{5}\]
done
clear
D)
\[\frac{1}{25}\]
done
clear
View Answer play_arrow
question_answer 62) The area under acceleration-time graph gives
A)
distance travelled
done
clear
B)
change in acceleration
done
clear
C)
force acting
done
clear
D)
change in velocity
done
clear
View Answer play_arrow
question_answer 63) A particle is displaced from a position\[(2\hat{i}-\hat{j}+\hat{k})\]to another position \[(3\hat{i}+2\hat{j}-2\hat{k})\] under the action of the force of \[(2\hat{i}+\hat{j}-\hat{k}).\] The work done by the force in an arbitrary unit is
A)
8
done
clear
B)
10
done
clear
C)
12
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 64) From the top of tower, a stone is thrown up. It reaches the ground in \[{{t}_{1}}\]second. A second stone thrown down-with the same speed reaches the ground in \[{{t}_{2}}\]second. A third stone released from rest reaches the ground in \[{{t}_{3}}\]second. Then
A)
\[{{t}_{3}}=\frac{({{t}_{1}}+{{t}_{2}})}{2}\]
done
clear
B)
\[{{t}_{3}}=\sqrt{{{t}_{1}}{{t}_{2}}}\]
done
clear
C)
\[\frac{1}{{{t}_{3}}}=\frac{1}{{{t}_{1}}}-\frac{1}{{{t}_{2}}}\]
done
clear
D)
\[t_{3}^{2}=t_{2}^{2}-t_{1}^{2}\]
done
clear
View Answer play_arrow
question_answer 65) An object is projected at an angle of \[\text{4}{{\text{5}}^{\text{o}}}\] with the horizontal. The horizontal range and maximum height reached will be in the ratio :
A)
1: 2
done
clear
B)
2 :1
done
clear
C)
1 : 4
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 66) If the length of the seconds hand in a stop-clock is 3 cm, the angular velocity and linear velocity of the tip is
A)
\[0.2047\text{ }rad/s,\text{ }0.0314\text{ }m{{s}^{-1}}\]
done
clear
B)
\[0.2547\text{ }rad/s,\text{ }0.314\text{ }m{{s}^{-1}}\]
done
clear
C)
\[0.1472\text{ }rad/s,\text{ }0.06314\text{ }m{{s}^{-1}}\]
done
clear
D)
\[~0.1047\text{ }rad/s,\text{ }0.00314\text{ }m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 67) A player caught a cricket ball of mass 150 g moving at the rate of \[\text{20}\,\text{m}{{\text{s}}^{-1}}.\] If the catching process be completed in 0.1 s, the force of the blow exerted by the ball on the hands of the player is
A)
0.3 N
done
clear
B)
30 N
done
clear
C)
300 N
done
clear
D)
3000 N
done
clear
View Answer play_arrow
question_answer 68) A uniform metal chain is placed on a rough table such that one end of it hangs down over the edge of the table. When one-third of its length hangs over the edge, the chain starts sliding. Then, the coefficient of static friction is
A)
0.3 N
done
clear
B)
30N
done
clear
C)
300 N
done
clear
D)
3000 N
done
clear
View Answer play_arrow
question_answer 69)
Two masses M and M/2 are joined together by means of light inextensible string passed over a frictionless pulley as shown in the figure. When the bigger mass is released, the small one will ascend with an acceleration of
A)
\[\frac{g}{3}\]
done
clear
B)
\[\frac{3g}{2}\]
done
clear
C)
\[\frac{g}{2}\]
done
clear
D)
g
done
clear
View Answer play_arrow
question_answer 70) In elastic collision
A)
both momentum and kinetic energies are conserved
done
clear
B)
both momentum and kinetic energies are not conserved
done
clear
C)
only energy is conserved
done
clear
D)
only mechanical energy is conserved
done
clear
View Answer play_arrow
question_answer 71)
Three identical spheres, each of mass 1kg are kept as shown in figure below, touching each other, with their centres on a straight line. If their centres are marked P, Q, R respectively, the distance of centre of mass of the system from P is
A)
(a ) \[\frac{PQ+PR+QR}{3}\]
done
clear
B)
\[\frac{PQ+PR}{3}\]
done
clear
C)
\[\frac{PQ+QR}{3}\]
done
clear
D)
\[\frac{PR+QR}{3}\]
done
clear
View Answer play_arrow
question_answer 72) The moment of inertia of a thin rod of mass M and length L, about an axis perpendicular to the rod at a distance \[\frac{L}{4}\] from one end is
A)
\[\frac{M{{L}^{2}}}{6}\]
done
clear
B)
\[\frac{M{{L}^{2}}}{12}\]
done
clear
C)
\[\frac{7M{{L}^{2}}}{24}\]
done
clear
D)
\[\frac{7M{{L}^{2}}}{48}\]
done
clear
View Answer play_arrow
question_answer 73) A body rolls down an inclined plane. If ifs kinetic energy of rotation is 40% of its kinetic energy of translation, then the body is
A)
solid cylinder
done
clear
B)
solid sphere
done
clear
C)
disc
done
clear
D)
ring
done
clear
View Answer play_arrow
question_answer 74) Which of the following statements about the gravitational constant is true?
A)
It is a force
done
clear
B)
It has no unit
done
clear
C)
It has same value in all systems of units
done
clear
D)
It does not depend on the nature of the medium in which the bodies are kept
done
clear
View Answer play_arrow
question_answer 75) Two identical solid copper spheres of radius R are placed in contact with each other. The gravitational attraction between them is proportional to
A)
\[{{R}^{2}}\]
done
clear
B)
\[{{R}^{-2}}\]
done
clear
C)
\[{{R}^{4}}\]
done
clear
D)
\[{{R}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 76) The modulus of elasticity is dimensionally equivalent to
A)
strain
done
clear
B)
force
done
clear
C)
stress
done
clear
D)
coefficient of viscosity
done
clear
View Answer play_arrow
question_answer 77) Radius of an air bubble at the bottom of the lake is r and it becomes 2r when the air bubble rises to the top surface of the lake. If P cm of water be the atmospheric pressure, then the depth of the lake is
A)
2P
done
clear
B)
8P
done
clear
C)
4P
done
clear
D)
7P
done
clear
View Answer play_arrow
question_answer 78) A manometer connected to a closed tap reads \[4.5\times {{10}^{5}}\,\]Pa. When the tap is opened the reading of the manometer falls to \[4\times {{10}^{5}}\,\] Pa. Then the velocity of flow of water is
A)
\[7\,m{{s}^{-1}}\]
done
clear
B)
\[8\,m{{s}^{-1}}\]
done
clear
C)
\[9\,m{{s}^{-1}}\]
done
clear
D)
\[10\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 79) The temperature of equal masses of three different liquids A, B and C are \[\text{12}{{\,}^{\text{o}}}\text{C,}\,\text{19}{{\,}^{\text{o}}}\text{C}\]and \[\text{28}{{\,}^{\text{o}}}\text{C}\]respectively. The temperature when A and B are mixed is \[\text{16}{{\,}^{\text{o}}}\text{C}\]and when B and C are mixed is \[\text{23}{{\,}^{\text{o}}}\text{C}\text{.}\] The temperature when A and C are mixed is
A)
\[18.2{{\,}^{o}}C\]
done
clear
B)
\[22{{\,}^{o}}C\]
done
clear
C)
\[20.2{{\,}^{o}}C\]
done
clear
D)
\[24.2{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 80) The time period of the seconds hand of a watch is
A)
1 h
done
clear
B)
1s
done
clear
C)
12 h
done
clear
D)
1 min
done
clear
View Answer play_arrow
question_answer 81) A particle starts SHM from the mean position. Its amplitude is a and total energy E. At one jg instant its kinetic energy is \[\text{3}\frac{\text{E}}{\text{4}}\text{.}\] Its 4 displacement at that instant is
A)
\[\frac{a}{\sqrt{2}}\]
done
clear
B)
\[\frac{a}{2}\]
done
clear
C)
\[\frac{a}{\sqrt{\left( \frac{3}{2} \right)}}\]
done
clear
D)
\[\frac{a}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 82) A particle executes linear simple harmonic motion with an amplitude of 2 cm. When the particle is at 1 cm from the mean position the magnitude of its velocity is equal to that of its acceleration. Then its time period in second is
A)
\[\frac{1}{2\pi \sqrt{3}}\]
done
clear
B)
\[2\pi \sqrt{3}\]
done
clear
C)
\[\frac{2\pi }{\sqrt{3}}\]
done
clear
D)
\[\frac{\sqrt{3}}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 83) A closed organ pipe and an open organ pipe are tuned to the same fundamental frequency. The ratio of their lengths is
A)
1:1
done
clear
B)
2:1
done
clear
C)
1: 4
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 84) An observer standing near the sea shore/min. If the wavelength of the water wave is 10 m, then the velocity of water wave is
A)
\[540\,m{{s}^{-1}}\]
done
clear
B)
\[5.4\,m{{s}^{-1}}\]
done
clear
C)
\[0.184\,m{{s}^{-1}}\]
done
clear
D)
\[9\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 85) A set of 24 tuning forks are so arranged that each gives 6 beats/s with the previous one. If the frequency of the last tuning fork is double that of the first, frequency of the second tuning fork is
A)
138 Hz
done
clear
B)
132 Hz
done
clear
C)
144 Hz
done
clear
D)
276 Hz
done
clear
View Answer play_arrow
question_answer 86) The electrostatic field due to a charged conductor just outside the conductor is
A)
zero and parallel to the surface at every point inside the conductor
done
clear
B)
zero and is normal to the surface at every point inside the conductor
done
clear
C)
parallel to the surface at every point and zero inside the conductor
done
clear
D)
normal to the surface at every point and zero inside the conductor
done
clear
View Answer play_arrow
question_answer 87) A point charge + q is placed at the midpoint of a cube of side a. The electric flux emerging from the cube is
A)
zero
done
clear
B)
\[\frac{3q{{a}^{2}}}{{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{{{\varepsilon }_{0}}}{4q{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 88)
Figure below shows four plates each of area A and separated from one another by a distance d.
A)
\[\frac{{{\varepsilon }_{0}}A}{d}\]
done
clear
B)
\[\frac{2{{\varepsilon }_{0}}A}{d}\]
done
clear
C)
\[\frac{3{{\varepsilon }_{0}}A}{d}\]
done
clear
D)
\[\frac{4{{\varepsilon }_{0}}A}{d}\]
done
clear
View Answer play_arrow
question_answer 89) A soap bubble is charged to a potential of 16 V. Its radius is, then doubled. The potential of the bubble now will be
A)
16 V
done
clear
B)
8 V
done
clear
C)
4 V
done
clear
D)
2 V
done
clear
View Answer play_arrow
question_answer 90) A \[10\,\Omega \]electric heater operates on a 110 V line. The rate at which heat is developed in watts is
A)
1310 W
done
clear
B)
670 W
done
clear
C)
810 W
done
clear
D)
1210 W
done
clear
View Answer play_arrow
question_answer 91) For a certain thermocouple, if the temperature of the cold junction is \[\text{0}{{\,}^{\text{o}}}\text{C,}\] the neutral temperature and inversion temperatures are \[285{{\,}^{\text{o}}}\text{C}\]and \[570{{\,}^{\text{o}}}\text{C}\]respectively. If the cold junction is brought to \[\text{10}{{\,}^{\text{o}}}\text{C,}\] then the new neutral and inversion temperatures are respectively
A)
\[285{{\,}^{o}}C\] and \[560{{\,}^{o}}C\]
done
clear
B)
\[285{{\,}^{o}}C\]and \[570{{\,}^{o}}C\]
done
clear
C)
\[295{{\,}^{o}}C\]and \[560{{\,}^{o}}C\]
done
clear
D)
\[275{{\,}^{o}}C\]and \[560{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 92) In which of the following substances does resistance decrease with increase in temperature?
A)
Copper
done
clear
B)
Carbon
done
clear
C)
Constantan
done
clear
D)
Silver
done
clear
View Answer play_arrow
question_answer 93) Resistors P and Q are connected in the gaps of the meter bridge. The balancing point is obtained \[\frac{1}{3}m\] from the zero end. If a\[6\,\Omega \] resistance is connected in series with P the balance point shifts to \[\frac{2}{3}m\] from the same end. P and Q are
A)
4, 2
done
clear
B)
2, 4
done
clear
C)
bothand
done
clear
D)
neithernor
done
clear
View Answer play_arrow
question_answer 94)
The currents \[{{i}_{1}}\] and \[{{i}_{2}}\] through the resistors \[{{R}_{1}}(=10\,\Omega )\] and\[{{R}_{2}}(=30\,\Omega )\] in the circuit diagram with \[{{E}_{1}}=3V,\,{{E}_{2}}=3V\] and \[{{E}_{3}}=2\,V\] are respectively
A)
0.2 A, 0.1 A
done
clear
B)
0.4 A, 0.2 A
done
clear
C)
0.1 A, 0.2 A
done
clear
D)
0.2 A, 0.4 A
done
clear
View Answer play_arrow
question_answer 95) An \[\alpha -\] particle with a specific charge of \[2.5\times {{10}^{7}}C-k{{g}^{-1}}\] moves with a speed of \[2\times {{10}^{5}}\,m{{s}^{-1}}\] in a perpendicular magnetic field of 0.05 T. Then the radius of the circular path described by it is
A)
8 cm
done
clear
B)
4 cm
done
clear
C)
16 cm
done
clear
D)
2 cm
done
clear
View Answer play_arrow
question_answer 96) A milliammeter of range 0 - 30 mA has internal resistance of \[20\,\Omega .\] The resistance to be connected in series to convert it into a voltmeter of maximum reading 3V is
A)
490
done
clear
B)
800
done
clear
C)
400
done
clear
D)
300
done
clear
View Answer play_arrow
question_answer 97) A straight conductor of length \[l\] carrying a current \[I,\] is bent in the form of a semicircle. The magnetic field (in tesla) at the centre of the semicircle is
A)
\[\frac{{{\pi }^{2}}I}{l}\times {{10}^{-7}}\]
done
clear
B)
\[\frac{\pi I}{l}\times {{10}^{-7}}\]
done
clear
C)
\[\frac{\pi I}{{{l}^{2}}}\times {{10}^{-7}}\]
done
clear
D)
\[\frac{\pi {{I}^{2}}}{l}\times {{10}^{-7}}\]
done
clear
View Answer play_arrow
question_answer 98) A coil having an inductance of 0.5 H carries a current which is uniformly varying from 0 to 10 A in 2 s. The emf (in volts) generated in the coil is
A)
10
done
clear
B)
5
done
clear
C)
2.5
done
clear
D)
1.25
done
clear
View Answer play_arrow
question_answer 99) If an alternating voltage is represented as \[E=141\,\sin (628\,t),\] then the rms value of the voltage and the frequency are respectively
A)
141V, 628 Hz
done
clear
B)
100 V, 50 Hz
done
clear
C)
100 V, 100 Hz
done
clear
D)
141 V, 100 Hz
done
clear
View Answer play_arrow
question_answer 100) A step-down transformer is used on a 1000 V line to deliver 20 A at 120 V at the secondary coil. If the efficiency of the transformer is 80%, the current drawn from the line is
A)
3 A
done
clear
B)
\[30\,A\]
done
clear
C)
\[0.3\,A\]
done
clear
D)
\[2.4\,A\]
done
clear
View Answer play_arrow
question_answer 101) A liquid is in equilibrium with its vapour at its boiling point. On the average, the molecules in two phases have equal
A)
inter- molecular forces
done
clear
B)
potential energy
done
clear
C)
kinetic energy
done
clear
D)
total energy
done
clear
View Answer play_arrow
question_answer 102) For the chemical reaction the amount of\[{{X}_{3}}Y\] at equilibrium is affected by
A)
temperature and pressure
done
clear
B)
temperature only
done
clear
C)
pressure only
done
clear
D)
temperature, pressure and catalyst
done
clear
View Answer play_arrow
question_answer 103) The pair of compound which cannot exists together in solution is
A)
\[NaHC{{O}_{3}}\]and \[NaOH\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]and \[NHC{{O}_{3}}\]
done
clear
C)
\[~N{{a}_{2}}C{{O}_{3}}\] and\[NaOH\]
done
clear
D)
\[NaHC{{O}_{3}}\]and x
done
clear
View Answer play_arrow
question_answer 104) The compound will react most readily with \[NaOH\] to form methanol is
A)
\[{{(C{{H}_{3}})}_{4}}{{N}^{+}}{{I}^{-}}\]
done
clear
B)
\[C{{H}_{3}}OC{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}{{S}^{+}}{{I}^{-}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 105) Which has maximum number of atoms?
A)
24 g of C
done
clear
B)
56 g of Fe
done
clear
C)
26 g of Al
done
clear
D)
108 g of Ag
done
clear
View Answer play_arrow
question_answer 106) In homogeneous catalysis
A)
the reactants, catalyst and products are in the same phase.
done
clear
B)
the catalyst and reactants are in the same phase.
done
clear
C)
the catalyst and products are in the same phase.
done
clear
D)
the reactants and products are in the same phase.
done
clear
View Answer play_arrow
question_answer 107) Which of the following has the smallest number of molecules?
A)
0.1 mole of \[C{{O}_{2}}\] gas
done
clear
B)
11.2L of \[C{{O}_{2}}\]gas at STP
done
clear
C)
22 got \[C{{O}_{2}}\]gas
done
clear
D)
\[22.4\times {{10}^{3}}mL\]of\[C{{O}_{2}}\]gas at STP
done
clear
View Answer play_arrow
question_answer 108) \[4.6\times {{10}^{22}}\]atoms of an element weigh 13.8g. The atomic weight of that element is
A)
290
done
clear
B)
180
done
clear
C)
34.4
done
clear
D)
10.4
done
clear
View Answer play_arrow
question_answer 109) The decomposition of hydrogen peroxide can be slowed by the addition of acetamide. The latter acts as a
A)
detainer
done
clear
B)
stopper
done
clear
C)
promoter
done
clear
D)
inhibitor
done
clear
View Answer play_arrow
question_answer 110) Which one of the following compounds has \[s{{p}^{2}}\]hybridisation?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}O\]
done
clear
D)
\[~CO\]
done
clear
View Answer play_arrow
question_answer 111) Identify the least stable ion amongst the following.
A)
\[L{{i}^{-}}\]
done
clear
B)
\[B{{e}^{-}}\]
done
clear
C)
\[~{{B}^{-}}\]
done
clear
D)
\[{{C}^{-}}\]
done
clear
View Answer play_arrow
question_answer 112) Number of lone pair (s) in \[XeO{{F}_{4}}\]is/are
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 113) The half-life period of radioactive element is 140 days. After 560 days, 1 gram of element will reduce to
A)
\[\frac{1}{2}g\]
done
clear
B)
\[\frac{1}{4}g\]
done
clear
C)
\[\frac{1}{8}g\]
done
clear
D)
\[\frac{1}{16}g\]
done
clear
View Answer play_arrow
question_answer 114) Which of the following is used in the treatment of blood cancer?
A)
\[{{I}^{131}}\]
done
clear
B)
\[{{P}^{32}}\]
done
clear
C)
\[Rn\]
done
clear
D)
\[{{I}^{127}}\]
done
clear
View Answer play_arrow
question_answer 115) The orbital angular momentum of an electron in \[2s\] orbital is
A)
\[+\frac{1}{2}\frac{h}{2\pi }\]
done
clear
B)
zero
done
clear
C)
\[\frac{h}{2\pi }\]
done
clear
D)
\[\sqrt{2}\frac{h}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 116) The starting material used in Solvas process are
A)
sodium sulphate
done
clear
B)
brine solution
done
clear
C)
camallite
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 117) The ionisation energy of hydrogen atom is 13.6 eV. What will be the ionisation energy of \[\text{H}{{\text{e}}^{\text{+}}}\]?
A)
\[13.6\text{ }eV\]
done
clear
B)
\[54.4\text{ }eV\]
done
clear
C)
\[122.4\text{ }eV\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 118) In Downs method for the extraction of sodium, the melting point of the electrolyte is lowered by adding
A)
potassium chloride
done
clear
B)
calcium chlpnde
done
clear
C)
both calcium chloride and potassium fluoride
done
clear
D)
potassium fluoride only
done
clear
View Answer play_arrow
question_answer 119) Calcium is obtained by
A)
electrolysis of molten \[CaC{{l}_{2}}\]
done
clear
B)
electrolysis of solution of \[CaC{{l}_{2}}\] in water
done
clear
C)
reduction of \[CaC{{l}_{2}}\] with carbon
done
clear
D)
roasting of lime stone
done
clear
View Answer play_arrow
question_answer 120) Which of the following is philosophers wool?
A)
\[ZnO\]
done
clear
B)
\[HgO\]
done
clear
C)
\[A{{g}_{2}}O\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 121) . The number of electrons and neutrons of an element is 18 and 20 respectively. Its mass number is
A)
2
done
clear
B)
17
done
clear
C)
37
done
clear
D)
38
done
clear
View Answer play_arrow
question_answer 122) The number of \[\alpha \] and \[\beta \] particles emitted in nuclear reaction \[{{\,}_{90}}T{{h}^{228}}\to {{\,}_{83}}B{{i}^{212}}\] are respectively
A)
4, 1
done
clear
B)
3, 7
done
clear
C)
8, 1
done
clear
D)
4, 7
done
clear
View Answer play_arrow
question_answer 123) 2g of mixture of CO and \[\text{C}{{\text{O}}_{\text{2}}}\]on reaction with excess \[{{\text{I}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]produced 2.54g of \[{{\text{I}}_{2}}.\]What would be the mass % of \[\text{C}{{\text{O}}_{\text{2}}}\]in the original mixture?
A)
60
done
clear
B)
30
done
clear
C)
70
done
clear
D)
35
done
clear
View Answer play_arrow
question_answer 124) When 10g of 90% pure lime stone is heated completely, the volume (in litres) of \[\text{C}{{\text{O}}_{\text{2}}}\]is liberated at STP is
A)
22.4
done
clear
B)
2.24
done
clear
C)
20.16
done
clear
D)
2.016
done
clear
View Answer play_arrow
question_answer 125) Which element has highest electronegativity ?
A)
F
done
clear
B)
He
done
clear
C)
Ne
done
clear
D)
Na
done
clear
View Answer play_arrow
question_answer 126) The first ionisation potential of Na, Mg, Al and Si are in the order
A)
Na < Mg > Al < Si
done
clear
B)
Na > Mg > Al > Si
done
clear
C)
Na < Mg < Al > Si
done
clear
D)
Na > Mg > Al < Si
done
clear
View Answer play_arrow
question_answer 127) Which is the chief ore of copper?
A)
Galena
done
clear
B)
Copper pyrites
done
clear
C)
Sphalerite
done
clear
D)
Siderite
done
clear
View Answer play_arrow
question_answer 128) Ferrous sulphate \[\text{(FeS}{{\text{O}}_{4}}\text{.7}{{\text{H}}_{2}}\text{O)}\] is known as
A)
vermillion
done
clear
B)
Glaubes salt
done
clear
C)
green vitriol
done
clear
D)
Mohrs salt
done
clear
View Answer play_arrow
question_answer 129) The correct order of radii is
A)
\[N<Be<B\]
done
clear
B)
\[{{F}^{-}}<{{O}^{2-}}<{{N}^{3-}}\]
done
clear
C)
\[Na<Li<K\]
done
clear
D)
\[F{{e}^{3+}}<F{{e}^{2+}}<F{{e}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 130) Law of constant composition is same as the law of
A)
conservation of mass
done
clear
B)
conservation of energy
done
clear
C)
multiple proportion
done
clear
D)
definite proportion
done
clear
View Answer play_arrow
question_answer 131) Which of the oxides does not react with both of an acid and alkali, is?
A)
\[ZnO\]
done
clear
B)
\[Sn{{O}_{2}}\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[BeO\]
done
clear
View Answer play_arrow
question_answer 132) \[\text{BC}{{\text{l}}_{\text{3}}}\]is a planar molecule, while \[\text{NC}{{\text{l}}_{3}}\]is pyramidal, because
A)
\[NCl\] bond is more covalent than \[BCl\] bond
done
clear
B)
nitrogen atom is smaller than boron atom
done
clear
C)
\[BCl\]bond is more polar than \[N-Cl\]bond
done
clear
D)
\[\text{BC}{{\text{l}}_{\text{3}}}\]has no lone pair of electrons but \[NC{{l}_{3}}\] has a lone pair of electrons
done
clear
View Answer play_arrow
question_answer 133) The oxidation state of C in \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]is
A)
\[+\text{ }6\]
done
clear
B)
\[-6\]
done
clear
C)
0
done
clear
D)
\[+\text{ }4\]
done
clear
View Answer play_arrow
question_answer 134) If molecular weight of \[KMn{{O}_{4}}\] is M, then its equivalent weight in acidic medium would be
A)
\[~M\]
done
clear
B)
\[\frac{M}{2}\]
done
clear
C)
\[\frac{M}{5}\]
done
clear
D)
\[\frac{M}{3}\]
done
clear
View Answer play_arrow
question_answer 135) A current of 12 ampere is passed through an electrolytic cell containing aqueous \[NiS{{O}_{4}}\] solution. Both Ni and \[{{H}_{2}}\]gas are formed at the cathode. The current efficiency is 60%. What is the mass of nickel deposited on the cathode per hour?
A)
7.883 g
done
clear
B)
3.941 g
done
clear
C)
5.91 g
done
clear
D)
2.645 g
done
clear
View Answer play_arrow
question_answer 136)
The correct IUPAC name of
A)
1-cyclopropyl cyclobutane
done
clear
B)
1,1-dicyclobutane
done
clear
C)
1-cyclobutane-1- cyclopropane
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 137) Geometrical isomerism is possible in
A)
acetone-oxime
done
clear
B)
isobutene
done
clear
C)
acetophenone-oxime
done
clear
D)
benzophenone-oxime
done
clear
View Answer play_arrow
question_answer 138) The cell reaction of a cell is \[Mg(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Cu(s)+M{{g}^{2+}}(aq).\]If the standard reduction potentials of Mg and Cu are \[-\text{ }2.37\] and \[+\text{ }0.34\,V\] respectively. The emf of the cell is
A)
\[2.03V\]
done
clear
B)
\[-2.03V\]
done
clear
C)
\[+2.71V\]
done
clear
D)
\[-2.71V\]
done
clear
View Answer play_arrow
question_answer 139) In the electrochemical reaction, \[2F{{e}^{3+}}+Zn\xrightarrow{{}}Z{{n}^{2+}}+2F{{e}^{2+}}\] increasing the concentration of\[F{{e}^{2+}}\]
A)
increases cell emf
done
clear
B)
increases the current flow
done
clear
C)
decrease the cell emf
done
clear
D)
alter the pH of the solution
done
clear
View Answer play_arrow
question_answer 140) Aluminium is obtained by
A)
reducing \[A{{l}_{2}}{{O}_{3}}\] with coke
done
clear
B)
electrolysing \[A{{l}_{2}}{{O}_{3}}\]dissolved in \[N{{a}_{3}}Al{{F}_{6}}\]
done
clear
C)
reducing \[A{{l}_{2}}{{O}_{3}}\]with chromium
done
clear
D)
heating alumina with cryolite
done
clear
View Answer play_arrow
question_answer 141) In the reaction, \[3A+2B\xrightarrow{{}}2C,\]the equilibrium constant \[{{K}_{c}}\]is given by
A)
\[\frac{[3A]\times [2B]}{[C]}\]
done
clear
B)
\[\frac{{{[A]}^{3}}\times [B]}{[C]}\]
done
clear
C)
\[\frac{{{[C]}^{2}}}{{{[A]}^{3}}\times {{[B]}^{2}}}\]
done
clear
D)
\[\frac{[C]}{[3A]\times [2B]}\]
done
clear
View Answer play_arrow
question_answer 142) 20 volume \[{{H}_{2}}{{O}_{2}}\]solution has a strength of about
A)
30%
done
clear
B)
6%
done
clear
C)
3%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 143) Permanent hardness can be removed by adding
A)
\[C{{l}_{2}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[Ca(OCl)Cl\]
done
clear
D)
\[{{K}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 144) \[{{N}_{2}}\]and\[{{H}_{2}}\]in 1 : 3 molar ratio are heated in a closed container having a catalyst. When the following equilibrium \[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\] is attained, the total pressure is 10 atm and mole fraction of \[N{{H}_{3}}\] is 0.60. The equilibrium constant \[{{K}_{p}}\] for dissociation of \[N{{H}_{3}}\] is
A)
\[1.333\text{ }at{{m}^{-2}}\]
done
clear
B)
\[0.75\text{ }at{{m}^{2}}\]
done
clear
C)
\[0.75\text{ }at{{m}^{-2}}\]
done
clear
D)
\[1.333\text{ }at{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 145) According to Le-Chatelier principle, adding heat a solid and liquid in equilibrium will cause the
A)
amount of solid to decrease
done
clear
B)
amount of liquid to decrease
done
clear
C)
temperature to rise
done
clear
D)
temperature to fall
done
clear
View Answer play_arrow
question_answer 146) What are the products obtained when ammonia is reacted with excess chlorine?
A)
\[{{N}_{2}}\]and \[NC{{l}_{3}}\]
done
clear
B)
\[{{N}_{2}}\]and \[HCl\]
done
clear
C)
\[{{N}_{2}}\] and \[N{{H}_{4}}Cl\]
done
clear
D)
\[NC{{l}_{3}}\]and \[HCl\]
done
clear
View Answer play_arrow
question_answer 147) Reaction of \[\text{HN}{{\text{O}}_{\text{3}}}\]with I, S, P and C gives respectively
A)
\[~HI{{O}_{3}},{{H}_{2}}S{{O}_{4}},{{H}_{3}}P{{O}_{4}}\]and \[C{{O}_{2}}\]
done
clear
B)
\[HI{{O}_{3}},\text{ }{{H}_{2}}S{{O}_{4}},\text{ }{{H}_{3}}P{{O}_{3}},\]and\[C{{O}_{2}}\]
done
clear
C)
\[HI{{O}_{2}},\text{ }{{H}_{2}}S{{O}_{4}},\text{ }{{H}_{3}}P{{O}_{4}},\]and CO
done
clear
D)
\[{{I}_{2}}{{O}_{5}},S{{O}_{2}},{{P}_{2}}O,\]and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 148) When phenol is treated with \[CHC{{l}_{3}}\]and NaOH the product formed is
A)
benzaldehyde
done
clear
B)
salicyaldehyde
done
clear
C)
salicylic acid
done
clear
D)
benzoicacid
done
clear
View Answer play_arrow
question_answer 149) The Kolbes electrolysis proceeds via
A)
nucleophilic substitution mechanism
done
clear
B)
electrophilic addition mechanism
done
clear
C)
free radical mechanism
done
clear
D)
electrophilic substitution reaction
done
clear
View Answer play_arrow
question_answer 150) Pure methane can be produced by
A)
Wurtz reaction
done
clear
B)
Kolbes electrolytic method
done
clear
C)
soda lime decarboxylation
done
clear
D)
reduction with \[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 151) Daltons law of partial pressure is not applicable to
A)
\[{{O}_{2}}+{{O}_{3}}\]
done
clear
B)
\[CO+C{{O}_{2}}\]
done
clear
C)
\[N{{H}_{3}}+HCl\]
done
clear
D)
\[I+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 152) Positive deviation from ideal behaviour takes place because of
A)
molecular interaction between atom and\[\frac{PV}{nRT}>1\]
done
clear
B)
molecular interaction between atom and \[\frac{PV}{nRT}<1\]
done
clear
C)
finite size of atoms and \[\frac{PV}{nRT}>1\]
done
clear
D)
finite size of atoms and\[\frac{PV}{nRT}<1\]
done
clear
View Answer play_arrow
question_answer 153) Solubility of \[AgCl\] at \[20{{\,}^{o}}C\] is \[1.435\times {{10}^{-3}}g/L.\] The solubility product of \[AgCl\] is
A)
\[1\times {{10}^{-5}}\]
done
clear
B)
\[1\times {{10}^{-10}}\]
done
clear
C)
\[1.435\times {{10}^{-5}}\]
done
clear
D)
\[108\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 154) At\[\text{30}{{\,}^{\text{o}}}\text{C}\] the solubility of \[A{{g}_{2}}C{{O}_{3}}({{K}_{sp}}=8\times {{10}^{-12}})\] would be greatest in one litre of
A)
\[0.05M\,N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[0.05\,M\,AgN{{O}_{3}}\]
done
clear
C)
pure water
done
clear
D)
\[0.05\,N\,N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 155) Dry bleach is done by
A)
\[{{O}_{3}}\]
done
clear
B)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 156) The formation of colloid from suspension is
A)
peptisation
done
clear
B)
condensation
done
clear
C)
sedimentation
done
clear
D)
fragmentation
done
clear
View Answer play_arrow
question_answer 157) Which of the following has maximum value of flocculating power?
A)
\[P{{b}^{2+}}\]
done
clear
B)
\[P{{b}^{4+}}\]
done
clear
C)
\[S{{r}^{2+}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 158) Alums are used for
A)
tanning of leather
done
clear
B)
coagulation of blood
done
clear
C)
purification of water
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 159) Bleaching action of bleaching powder is due to the liberation of
A)
\[{{O}_{2}}\]
done
clear
B)
\[OC{{l}^{-}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 160) Which of the following can behave as an acid as well as base?
A)
\[HCl\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 161) Ammonium ion is
A)
a conjugate acid
done
clear
B)
a conjugate base
done
clear
C)
neither acid nor a base
done
clear
D)
both an acid and a base
done
clear
View Answer play_arrow
question_answer 162) In the titration of \[N{{a}_{2}}C{{O}_{3}}\]and \[HCl,\]the indicator used is
A)
methyl orange
done
clear
B)
methylene blue
done
clear
C)
phenolphthalein
done
clear
D)
litmus
done
clear
View Answer play_arrow
question_answer 163) 74.5 g of a metallic chloride contains 35.5 g of chlorine, the equivalent weight of the metal is
A)
19.5
done
clear
B)
35.5
done
clear
C)
39
done
clear
D)
78.0
done
clear
View Answer play_arrow
question_answer 164) Catalyst used in dimerisation of acetylene to prepare chloroprene is
A)
\[HgS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
C)
\[C{{u}_{2}}C{{l}_{2}}+N{{H}_{4}}Cl\]
done
clear
D)
\[C{{u}_{2}}C{{l}_{2}}+N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 165) lodoform can be obtained on warming NaOH and iodine with
A)
\[C{{H}_{3}}-C{{H}_{2}}-CH(OH)C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CH-\overset{O}{\mathop{\overset{|\,\,|}{\mathop{C}}\,}}\,-{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{O}{\mathop{\underset{|\,\,|}{\mathop{C}}\,}}\,-OC{{H}_{3}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}CC{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 166) The dehydration of butan-1-ol gives
A)
1-butene as the main product
done
clear
B)
2-butene as the main product
done
clear
C)
equal amounts of 1-butene and 2-butene
done
clear
D)
2-methyl propene
done
clear
View Answer play_arrow
question_answer 167) Methanol cannot be dried with anhydrous \[\text{CaC}{{\text{l}}_{\text{2}}}\]because
A)
\[\text{CaC}{{\text{l}}_{\text{2}}}\] dissolves in it
done
clear
B)
it is not good dehydrating agent
done
clear
C)
it forms as solid \[CaC{{l}_{2}}.4\text{ }C{{H}_{3}}OH\]
done
clear
D)
it reacts with \[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 168) Excess of \[KI\]reacts with \[CuS{{O}_{4}}\]solution and then \[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]solution is added to it. Which of the statements is incorrect for this reaction?
A)
\[\text{C}{{\text{u}}_{\text{2}}}{{\text{I}}_{\text{2}}}\] is formed
done
clear
B)
\[\text{Cu}{{\text{I}}_{\text{2}}}\]is formed
done
clear
C)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]is oxidized
done
clear
D)
Evolved \[{{I}_{2}}\] is reduced
done
clear
View Answer play_arrow
question_answer 169) A compound X on heating gives a colourless gas. This residue is dissolved in water to obtain Y. Excess \[C{{O}_{2}}\]is bubbled through aqueous solution of Y when Z is formed. Z on gentle heating gives back X. The X is
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[Ca{{(HC{{O}_{3}})}_{2}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[NaHC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 170) Red P can be obtained by white P by
A)
Heating it with a catalyst, in an inert atmosphere
done
clear
B)
Distilling it in an inert atmosphere
done
clear
C)
Dissolving it in \[C{{S}_{2}}\]and crystallizing
done
clear
D)
Melting it and pouring the liquid into water
done
clear
View Answer play_arrow
question_answer 171) The basicity of \[{{H}_{3}}P{{O}_{4}}\] is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 172) \[N{{H}_{3}}\] has much higher boiling point than \[P{{H}_{3}}\] because
A)
\[N{{H}_{3}}\]has larger molecular weight
done
clear
B)
\[N{{H}_{3}}\] undergoes umbrella inversion
done
clear
C)
\[N{{H}_{3}}\] forms hydrogen bond
done
clear
D)
\[N{{H}_{3}}\]contains ionic bonds whereas \[P{{H}_{3}}\]contains covalent bonds
done
clear
View Answer play_arrow
question_answer 173) Ethers are isomeric with
A)
aldehyde
done
clear
B)
ketones
done
clear
C)
both aldehyde and ketones
done
clear
D)
alcohols
done
clear
View Answer play_arrow
question_answer 174) In Williamsons synthesis
A)
an alkyl halide is treated with sodium alkoxide
done
clear
B)
an alkyl halide is treated with sodium
done
clear
C)
an alcohol is heated with cone. \[{{H}_{2}}S{{O}_{4}}\]at \[\text{130}{{\,}^{\text{o}}}\text{C}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 175) \[\text{BC}{{\text{l}}_{3}}+{{H}_{2}}O\xrightarrow{{}}X,\]the products formed in the reaction are
A)
\[~{{B}_{2}}{{O}_{3}}+HOC1\]
done
clear
B)
\[{{H}_{3}}B{{O}_{3}}+HCl\]
done
clear
C)
\[{{B}_{2}}{{H}_{6}}+HCl\]
done
clear
D)
no reaction
done
clear
View Answer play_arrow
question_answer 176) The borax bead test the coloured ions give characteristic coloured beads due to formation of
A)
metal borates
done
clear
B)
metal metaborates
done
clear
C)
metal phosphates
done
clear
D)
metal tetraborates
done
clear
View Answer play_arrow
question_answer 177) The Cannizaro reaction is not given by
A)
trimethyl acetaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
benzaldehyde
done
clear
D)
formaldehyde
done
clear
View Answer play_arrow
question_answer 178) Self condensation of acetaldehyde, in the presence of dilute alkalies gives
A)
an acetal
done
clear
B)
an aldol
done
clear
C)
mesitylene
done
clear
D)
propionaldehyde
done
clear
View Answer play_arrow
question_answer 179) Which of the following is used in making printers ink, shoe polish, black varnish and paint?
A)
Lamp black
done
clear
B)
Bone black
done
clear
C)
Carbon black
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 180) Stephens reduction is used to prepare aldehyde from
A)
alcohol
done
clear
B)
alkyl cyanides
done
clear
C)
alkanones
done
clear
D)
acid chlorides
done
clear
View Answer play_arrow
question_answer 181) CO is poisnous gas, antidote for CO poisoning is
A)
carborundum
done
clear
B)
carbogen
done
clear
C)
corbonic acid
done
clear
D)
pure oxygen
done
clear
View Answer play_arrow
question_answer 182) Moderate electrical conductivity is shown by
A)
silica
done
clear
B)
graphite
done
clear
C)
diamond
done
clear
D)
carborundum
done
clear
View Answer play_arrow
question_answer 183) Iodine readily dissolves in potassium iodide solution giving
A)
\[{{I}^{-}}\]
done
clear
B)
\[K{{I}^{-}}\]
done
clear
C)
\[KI_{2}^{-}\]
done
clear
D)
\[K{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 184) Which among the following is paramagnetic?
A)
\[C{{l}_{2}}O\]
done
clear
B)
\[Cl{{O}_{2}}\]
done
clear
C)
\[C{{l}_{2}}{{O}_{7}}\]
done
clear
D)
\[C{{l}_{2}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 185) Given below are some statements concerning formic acid, which of them is true?
A)
It is weaker acid than acetic acid
done
clear
B)
It is reducing agent
done
clear
C)
When its calcium salt is heated, it forms a ketone
done
clear
D)
It is an oxidising agent
done
clear
View Answer play_arrow
question_answer 186) Acetic acid reacts with \[PC{{l}_{5}}\]to form
A)
\[C{{H}_{2}}ClCOOH\]
done
clear
B)
\[CHC{{l}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}COCl\]
done
clear
D)
\[C{{H}_{3}}COOCl\]
done
clear
View Answer play_arrow
question_answer 187) Which of the following gives oxalic acid?
A)
Heating of acetic acid
done
clear
B)
Action of nitric acid on glucose
done
clear
C)
Acidic hydrolysis of cyanogen
done
clear
D)
Strong heating of sodium formate
done
clear
View Answer play_arrow
question_answer 188) Sulphur does not exist as \[{{S}_{2}}\]molecule because
A)
it is less electronegative
done
clear
B)
it is not able to constitute pn - pn bonds
done
clear
C)
it has ability to exhibit catenation
done
clear
D)
of tendency to show variable oxidation states
done
clear
View Answer play_arrow
question_answer 189) Polyanion formation is maximum in
A)
nitrogen
done
clear
B)
sulphur
done
clear
C)
oxygen
done
clear
D)
boron
done
clear
View Answer play_arrow
question_answer 190) Oleum is
A)
castor oil
done
clear
B)
oil of vitriol
done
clear
C)
fuming \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 191) Which of the following is most reactive towards electrophilic nitration?
A)
Toluene
done
clear
B)
Benzene
done
clear
C)
Benzoicacid
done
clear
D)
Nitrobenzene
done
clear
View Answer play_arrow
question_answer 192) Benzene diazonium chloride on reaction with phenol in weakly basic medium gives
A)
diphenyl ether
done
clear
B)
p-hydroxy azobenzene
done
clear
C)
chlorobenzene
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 193) Hinsbergs reagent is
A)
\[{{C}_{6}}{{H}_{5}}COCl\]
done
clear
B)
\[C{{H}_{3}}COCl\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\]
done
clear
View Answer play_arrow
question_answer 194) Reaction of aniline with benzaldehyde is
A)
substitution
done
clear
B)
addition
done
clear
C)
condensation
done
clear
D)
polymerization
done
clear
View Answer play_arrow
question_answer 195) In the following reaction, X \[X\xrightarrow{\text{bromination}}Y\] \[\xrightarrow[+HCl]{NaN{{O}_{2}}}Z\xrightarrow[{{C}_{2}}{{H}_{5}}OH]{boiling}\]tribromo benzene X is
A)
benzoic acid
done
clear
B)
salicylic acid
done
clear
C)
phenol
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 196)
A)
\[NO_{3}^{-}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[{{I}^{-}}\]
done
clear
D)
\[B{{r}^{-}}\]
done
clear
View Answer play_arrow
question_answer 197) Nesslers reagent is used to detect
A)
\[CrO_{4}^{2-}\]
done
clear
B)
\[PO_{4}^{3-}\]
done
clear
C)
\[MnO_{4}^{-}\]
done
clear
D)
\[NH_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 198) Oxidation-number of sulphur in Caros acid is :
A)
+ 6
done
clear
B)
+ 4
done
clear
C)
+ 8
done
clear
D)
+ 7
done
clear
View Answer play_arrow
question_answer 199) Liquor ammonia is
A)
ammonium hydroxide
done
clear
B)
liquified ammonia gas
done
clear
C)
concentrated solution of \[N{{H}_{3}}\]in water
done
clear
D)
a solution of \[N{{H}_{3}}\]in alcohol
done
clear
View Answer play_arrow
question_answer 200) Glycerol, on heating with oxalic acid at \[110{{\,}^{o}}C\] gives
A)
ethanol
done
clear
B)
methanoic acid
done
clear
C)
ether
done
clear
D)
acetone
done
clear
View Answer play_arrow
question_answer 201) A force which acts against the achievement of highest possible level to population growth is known as
A)
population pressure
done
clear
B)
saturation level
done
clear
C)
carrying capacity
done
clear
D)
environmental resistance
done
clear
View Answer play_arrow
question_answer 202) Antibiotic flavicin is obtained from
A)
Aspergillus fiavus
done
clear
B)
Aspergillus clavatum
done
clear
C)
Streptomyces grieseus
done
clear
D)
Streptomyces fradiae
done
clear
View Answer play_arrow
question_answer 203) The crystal of lead zirconate is a key component of
A)
sonography
done
clear
B)
electrocardiography
done
clear
C)
electroencephalography
done
clear
D)
magnetoencephalography
done
clear
View Answer play_arrow
question_answer 204) Antifeedant property occurs in
A)
nicotine
done
clear
B)
azadiractin
done
clear
C)
rotenone
done
clear
D)
cinerin
done
clear
View Answer play_arrow
question_answer 205) Latest trend in plant disease control is
A)
chemical control
done
clear
B)
biological control
done
clear
C)
good manure and fertilizer
done
clear
D)
breeding for disease resistance
done
clear
View Answer play_arrow
question_answer 206) Silk is the secretion of
A)
cephalic glands
done
clear
B)
gastric glands
done
clear
C)
buccal glands
done
clear
D)
salivary glands
done
clear
View Answer play_arrow
question_answer 207) Endometrium is the lining of
A)
bladder
done
clear
B)
vagina
done
clear
C)
uterus
done
clear
D)
oviduct
done
clear
View Answer play_arrow
question_answer 208) Which animal has gone extinct in recent times in India?
A)
Acinonyx jubaCus
done
clear
B)
Antilope cervicapra
done
clear
C)
Rhinocerus unicomis
done
clear
D)
Panthera leo
done
clear
View Answer play_arrow
question_answer 209) What is true about cleavage in the fertilized egg in humans?
A)
It is meroblastic
done
clear
B)
It starts while the egg is in fallopian tube
done
clear
C)
It is identical to normal mitosis
done
clear
D)
It starts when the egg reaches in uterus
done
clear
View Answer play_arrow
question_answer 210) Climacteric is
A)
a phenomenon related to fruit ripening
done
clear
B)
the condition of a plant when all its fruits are almost ripe
done
clear
C)
the condition of a plant when most of its leaves have turned yellow
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 211) Ethyleneisa
A)
gaseous hormone
done
clear
B)
gaseous enzyme
done
clear
C)
liquid - gas mixture
done
clear
D)
solid hormone
done
clear
View Answer play_arrow
question_answer 212) When the pollen tube enters through the micropyle, it is termed as
A)
chalazogamy
done
clear
B)
mesogamy
done
clear
C)
porogamy
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 213) For self pollination, flower must be
A)
unisexual
done
clear
B)
bisexual
done
clear
C)
monosexual
done
clear
D)
asexual
done
clear
View Answer play_arrow
question_answer 214) Which is not a gonadal hormone?
A)
Progesterone
done
clear
B)
Testosrerone
done
clear
C)
Adrenalin
done
clear
D)
Estrogen
done
clear
View Answer play_arrow
question_answer 215) Cochlea of mammalian internal ear is concerned with
A)
hearing
done
clear
B)
balance of body posture
done
clear
C)
both and
done
clear
D)
perception changes of atmospheric Pressure
done
clear
View Answer play_arrow
question_answer 216) The pan of an eye which acts like a diaphragm of a photographic camera is
A)
pupil
done
clear
B)
iris
done
clear
C)
lens
done
clear
D)
cornea
done
clear
View Answer play_arrow
question_answer 217) The dendrite carries impulses
A)
towards the cyton
done
clear
B)
away from cyton
done
clear
C)
across the body
done
clear
D)
from one neuron to another
done
clear
View Answer play_arrow
question_answer 218) Cerebrospinal fluid is present
A)
beneath the pia mater
done
clear
B)
between piamater and arachnoid
done
clear
C)
between arachnoid and duramater
done
clear
D)
between duramater and cranium
done
clear
View Answer play_arrow
question_answer 219) Cranium is made up of
A)
8 bones
done
clear
B)
12 bones
done
clear
C)
10 bones
done
clear
D)
16 bones
done
clear
View Answer play_arrow
question_answer 220) The function of surfactant is/are
A)
facilitating lung expansion
done
clear
B)
maintaining the stable size of the alveoli
done
clear
C)
to reduce the surface tension on the alveoli
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 221) Two chief functions of the leaves are
A)
photosynthesis and respiration
done
clear
B)
photosynthesis and transpiration
done
clear
C)
transpiration and respiration
done
clear
D)
respiration and digestion
done
clear
View Answer play_arrow
question_answer 222) The most advanced type of inflorescence is
A)
corymb
done
clear
B)
catkin
done
clear
C)
spadix
done
clear
D)
capitulum
done
clear
View Answer play_arrow
question_answer 223) Carthamus tinctorius belongs to family
A)
Asteraceae
done
clear
B)
Solanaceae
done
clear
C)
Malvaceae
done
clear
D)
Fabaceae
done
clear
View Answer play_arrow
question_answer 224) The organism in which Krebs cycle does not occur in mitochondria is
A)
yeast
done
clear
B)
E. coli
done
clear
C)
Ulothrix
done
clear
D)
mould
done
clear
View Answer play_arrow
question_answer 225) The plant undergoes wilting when
A)
xylem is blocked
done
clear
B)
cambium is blocked
done
clear
C)
phloem is blocked
done
clear
D)
some roots are reduced in number
done
clear
View Answer play_arrow
question_answer 226) Stomata open at night and close during day in
A)
xerophytes
done
clear
B)
gametophytes
done
clear
C)
mesophytes
done
clear
D)
hydrophytes
done
clear
View Answer play_arrow
question_answer 227) Leucocytes (WBCs) are considered as true cells because
A)
they possess nucleus
done
clear
B)
they do not contain haemoglobin
done
clear
C)
they show great power of movement
done
clear
D)
they are responsible for phagocytic activity
done
clear
View Answer play_arrow
question_answer 228) Which of the following cytoplasmic granules contain histamine?
A)
Basophils
done
clear
B)
Acidophils
done
clear
C)
Eosinophils
done
clear
D)
Neutrophils
done
clear
View Answer play_arrow
question_answer 229) Refractory period of a muscle fibre in mammals is
A)
0.8001 sec
done
clear
B)
0.002 sec
done
clear
C)
0.004 sec
done
clear
D)
0.005 sec
done
clear
View Answer play_arrow
question_answer 230) During muscle contraction
A)
size of A bands remain same
done
clear
B)
size of H zone becomes smaller
done
clear
C)
size of T band decreases
done
clear
D)
diameter of fibre increases
done
clear
View Answer play_arrow
question_answer 231) During photosynthesis
A)
both \[C{{O}_{2}}\]and water get oxidized
done
clear
B)
both \[C{{O}_{2}}\]and water get reduced
done
clear
C)
water is reduced and \[C{{O}_{2}}\]is oxidized
done
clear
D)
\[C{{O}_{2}}\]gets reduced and water gets oxidized
done
clear
View Answer play_arrow
question_answer 232) The order of occurrence of the cytochromes in the \[{{F}_{1}}\]particle is
A)
\[cyt-b,cyt-c,cyt-acyt\text{ }{{a}_{3}}\]
done
clear
B)
\[cyt-c,cyt-b,cyt-a-cyt\text{ }{{a}_{3}}\]
done
clear
C)
\[cyt-a,\text{ }cyt-b,cyt-ccyt\text{ }{{a}_{3}}\]
done
clear
D)
\[cyt-,cyt-a,cyt-ccyt\text{ }b\]
done
clear
View Answer play_arrow
question_answer 233) Dispersal of fruits in Opium (poppy) occurs through shaking by wind by
A)
explosive mechanism
done
clear
B)
parachute mechanism
done
clear
C)
censer mechanism
done
clear
D)
jacular mechanism
done
clear
View Answer play_arrow
question_answer 234) Storage leaves occur in
A)
Allium
done
clear
B)
Zizyphus
done
clear
C)
TriCicum
done
clear
D)
Trapa
done
clear
View Answer play_arrow
question_answer 235) Tube-within-a-tube body plan is shown by
A)
coelenterates
done
clear
B)
platyhelminthes
done
clear
C)
aschelminthes (nemathelminthes)
done
clear
D)
porifers
done
clear
View Answer play_arrow
question_answer 236) True coelom appeared first in the course of evolution in
A)
Aschelminthes
done
clear
B)
Chordata
done
clear
C)
Echinodermata
done
clear
D)
Annelida
done
clear
View Answer play_arrow
question_answer 237) Which of the following is not found in vertebrates?
A)
Bilateral symmetry
done
clear
B)
Gill opening
done
clear
C)
Body scales
done
clear
D)
Cnidoblasts
done
clear
View Answer play_arrow
question_answer 238) The cell organelles are found in
A)
bacterial cells
done
clear
B)
cyanobacterial cells
done
clear
C)
prokaryotic cells
done
clear
D)
eukaryoric cells
done
clear
View Answer play_arrow
question_answer 239) Intracellular compartments are not found in the cells of
A)
lower plants
done
clear
B)
prokaryotes
done
clear
C)
higher plants
done
clear
D)
eukaryotes
done
clear
View Answer play_arrow
question_answer 240) Spindle chromosomes have
A)
centriole
done
clear
B)
kinetochore
done
clear
C)
chromocentre
done
clear
D)
chromomere
done
clear
View Answer play_arrow
question_answer 241) Adrenal gland is derived from
A)
ectoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
both and
done
clear
View Answer play_arrow
question_answer 242) Which endocrine gland becomes inactive in old age?
A)
Adrenal
done
clear
B)
Pineal
done
clear
C)
Thymus
done
clear
D)
Pituitary
done
clear
View Answer play_arrow
question_answer 243) The process by which chloride ions pass into RBC and bicarbonate ions pass out is called
A)
bicarbonate shift
done
clear
B)
Chloride shift
done
clear
C)
buffer system
done
clear
D)
enzyme shift
done
clear
View Answer play_arrow
question_answer 244) Squeezing of leucocytes out from the endothelium of capillaries to fight foreign agents is known as
A)
haemolysis
done
clear
B)
diapaedesis
done
clear
C)
phagocytosis
done
clear
D)
rouleaux
done
clear
View Answer play_arrow
question_answer 245) Which organ is considered as Graveyard of RBC where most of them are destroyed by macrophages?
A)
Red bone marrow
done
clear
B)
Spleen
done
clear
C)
Kidney
done
clear
D)
Intestine
done
clear
View Answer play_arrow
question_answer 246) When one organism is benefited without affecting the other, it is called
A)
parasitism
done
clear
B)
commensalism
done
clear
C)
saprophyrism
done
clear
D)
symbiosis
done
clear
View Answer play_arrow
question_answer 247) A branch of science in which forest crop can be grown in desired pattern is
A)
Histology
done
clear
B)
Horticulture
done
clear
C)
Pisiculture
done
clear
D)
Siliviculture
done
clear
View Answer play_arrow
question_answer 248) Which of the following factor is biotic?
A)
Photoperiod
done
clear
B)
\[C{{O}_{2}}\]content to the soil
done
clear
C)
Texture and porosity of soil
done
clear
D)
Rainfall
done
clear
View Answer play_arrow
question_answer 249) The pyramid of energy in a forest ecosystem is
A)
always upright
done
clear
B)
always inverted
done
clear
C)
both and
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 250) Lichens are important in the studies on atmospheric pollution because they
A)
can grow in polluted atmosphere
done
clear
B)
can readily multiply in polluted atmosphere
done
clear
C)
efficiently purify the atmosphere
done
clear
D)
are very sensitive to pollutants tike sulphur dioxide
done
clear
View Answer play_arrow
question_answer 251) 9:3:3:1 ratio is modified to 9 : 7 ratio due to
A)
complementary gene
done
clear
B)
epistatic gene
done
clear
C)
hypostatic gene
done
clear
D)
supplementary gene
done
clear
View Answer play_arrow
question_answer 252) The largest quantity of air that can be expired after a maximum inspiratory effort is
A)
residual volume
done
clear
B)
tidal volume
done
clear
C)
vital capacity of lung
done
clear
D)
lung volume
done
clear
View Answer play_arrow
question_answer 253) Shifting of ammonotelism to ureotelism is seen in
A)
fishes
done
clear
B)
frog
done
clear
C)
protopterus
done
clear
D)
snake
done
clear
View Answer play_arrow
question_answer 254) The amount of liquid filtered by glomeruli of kidney in 24 hours period is
A)
170 L
done
clear
B)
100 L
done
clear
C)
200-250 cc
done
clear
D)
500-1000 cc
done
clear
View Answer play_arrow
question_answer 255) The map distance between the genes A and B is 3 units, between B and C is 10 units and between C and A is 7 units. The order of the genes in a linkage map constructed on the above data would perhaps be
A)
A, B, C
done
clear
B)
A, C, B
done
clear
C)
B, C, A
done
clear
D)
B, A, C
done
clear
View Answer play_arrow
question_answer 256) In the lactose operon of Escherichia coli what is the function of promoter?
A)
Binding of Gyrase enzyme
done
clear
B)
Binding of RNA polymerase
done
clear
C)
Codes for RNA polymerase
done
clear
D)
Processing of messenger RNA
done
clear
View Answer play_arrow
question_answer 257) One of the following is sex-influenced character
A)
Appearance of beard
done
clear
B)
Baldness in male
done
clear
C)
Docile behaviour of female
done
clear
D)
Low BMR in female
done
clear
View Answer play_arrow
question_answer 258) Polymerase chain reaction is most useful in
A)
DNA synthesis
done
clear
B)
DNA amplification
done
clear
C)
protein synthesis
done
clear
D)
amino acid synthesis
done
clear
View Answer play_arrow
question_answer 259) In mitosis the duplication of chromosomes occurs during
A)
early prophase
done
clear
B)
late prophase
done
clear
C)
interphase
done
clear
D)
late telophase
done
clear
View Answer play_arrow
question_answer 260) Bivalents in meiosis are
A)
tetrad
done
clear
B)
pairs of non-homologous chromosomes
done
clear
C)
pairs of several chromatids
done
clear
D)
pairs of homologous chromosomes
done
clear
View Answer play_arrow
question_answer 261) Which one of the following is also called halophiles?
A)
Eubacteria
done
clear
B)
Actinomycetes
done
clear
C)
Archaebacteria
done
clear
D)
Cyanobacteria
done
clear
View Answer play_arrow
question_answer 262) Sex factor in bacteria is
A)
F- replicon
done
clear
B)
chromosomal replicon
done
clear
C)
RNA
done
clear
D)
sex pillies
done
clear
View Answer play_arrow
question_answer 263) Vascular bundles of roots are
A)
conjoint
done
clear
B)
concentric
done
clear
C)
bicollateral
done
clear
D)
radial
done
clear
View Answer play_arrow
question_answer 264) Ectophloic siphonostele is found in
A)
Osmunda and Equisetum
done
clear
B)
Marsilea and Botrychium
done
clear
C)
Adiantum and Cucurbitaceae
done
clear
D)
Dicksonia and Maiden hair fern
done
clear
View Answer play_arrow
question_answer 265) Adipocytes are mainly found in
A)
bones
done
clear
B)
cartilages
done
clear
C)
connective tissue
done
clear
D)
nerves
done
clear
View Answer play_arrow
question_answer 266) Arbor-vitae is composed of
A)
grey matter
done
clear
B)
neurogleal cells
done
clear
C)
white matter
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 267) The main difference between white and yellow Fibres is of
A)
protein
done
clear
B)
color of fibres
done
clear
C)
both and
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 268) Lenticular transpiration takes place in
A)
fruits
done
clear
B)
woody stems
done
clear
C)
leaves
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 269) In which of the following process 36 ATP molecules are produced by per hexose molecule?
A)
Glycolysis
done
clear
B)
Krebs cycle
done
clear
C)
Direct oxidation pathway
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 270) The value of RQ at compensation point is
A)
unity
done
clear
B)
infinity
done
clear
C)
>1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 271) The vitamin present in Coenzyme is
A)
\[{{B}_{2}}\]
done
clear
B)
niacin
done
clear
C)
pyridoxine
done
clear
D)
pantothenic acid
done
clear
View Answer play_arrow
question_answer 272) Symbiotic bacteria present in intestine of most primates, which synthesize certain vitamins are
A)
Entamoeba histolytica
done
clear
B)
Entamoeba coli
done
clear
C)
Entamoeba gingivalis
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 273) In which form \[C{{O}_{2}}\]is mostly carried by blood?
A)
Bicarbonates
done
clear
B)
Carbonic acid
done
clear
C)
Carbamino compound
done
clear
D)
Carboxyhaemoglobin
done
clear
View Answer play_arrow
question_answer 274) Which artery is absent in frog?
A)
Right systemic arch
done
clear
B)
Phrenic artery
done
clear
C)
Carotid artery
done
clear
D)
Renal artery
done
clear
View Answer play_arrow
question_answer 275) The number of RBCs in man increases if he lives at a higher altitude, this is because
A)
there is more oxygen at the mountains
done
clear
B)
there is less oxygen at mountains
done
clear
C)
more heat is required to be produced in the body for keeping warm
done
clear
D)
there are no germs in the air in mountain
done
clear
View Answer play_arrow
question_answer 276) Foramen magnum is situated in
A)
buccal cavity
done
clear
B)
base of skull
done
clear
C)
left auricle
done
clear
D)
vertebrae
done
clear
View Answer play_arrow
question_answer 277) The genes and splenium in brain are associated with
A)
cerebellum
done
clear
B)
cerebrum
done
clear
C)
medulla oblongata
done
clear
D)
vermis
done
clear
View Answer play_arrow
question_answer 278) Scala vestibuli is connected with
A)
fenestra rotundus
done
clear
B)
fenestra ovalis
done
clear
C)
scala tympani
done
clear
D)
scala media
done
clear
View Answer play_arrow
question_answer 279) The friction between the eyelids and the cornea is avoided by the secretion of
A)
lachrymal glands
done
clear
B)
conjunctiva and eyelids
done
clear
C)
hardarian glands
done
clear
D)
Meibomian glands
done
clear
View Answer play_arrow
question_answer 280) Embryo sac is
A)
microgametophyte
done
clear
B)
microsporangium
done
clear
C)
megagametophyte
done
clear
D)
megasporangium
done
clear
View Answer play_arrow
question_answer 281) Sprouting of potato can be prevented in storage by
A)
maleic hydrazide
done
clear
B)
gibberellins
done
clear
C)
indole acetic acid
done
clear
D)
cytokinins
done
clear
View Answer play_arrow
question_answer 282) Tendrils exhibit/twining of tendrils is due to
A)
thigmotropism
done
clear
B)
seismonasty
done
clear
C)
heliotropism
done
clear
D)
diageotropism
done
clear
View Answer play_arrow
question_answer 283) Flowering dependent on cold treatment is
A)
cryotherapy
done
clear
B)
cryogenics
done
clear
C)
cryoscopy
done
clear
D)
vernalisation
done
clear
View Answer play_arrow
question_answer 284) Noncleidoic eggs occur in
A)
birds
done
clear
B)
Fish
done
clear
C)
reptiles
done
clear
D)
platypus
done
clear
View Answer play_arrow
question_answer 285) Human sperm was discovered by
A)
Leeuwenhoek
done
clear
B)
Aristotle
done
clear
C)
Graaf
done
clear
D)
Pander
done
clear
View Answer play_arrow
question_answer 286) Sunken stomata occur in
A)
xerophytes
done
clear
B)
hydrophytes
done
clear
C)
mesophytes
done
clear
D)
opsanophytes
done
clear
View Answer play_arrow
question_answer 287) Soil carried by gravity is
A)
alluvial
done
clear
B)
eolian
done
clear
C)
colluvial
done
clear
D)
glacial
done
clear
View Answer play_arrow
question_answer 288) Metha Jahar which is useful in rheumatism is obtained from
A)
Aconitum napellus
done
clear
B)
Colchicum
done
clear
C)
Exogonium
done
clear
D)
Licorice
done
clear
View Answer play_arrow
question_answer 289) Long fibres of cotton seed are known as
A)
coir
done
clear
B)
fuzz
done
clear
C)
lint
done
clear
D)
flax
done
clear
View Answer play_arrow
question_answer 290) The pioneer country in the production of fuel alcohol is
A)
Saudi Arabia
done
clear
B)
Iran, Iraq
done
clear
C)
Brazil
done
clear
D)
Japan
done
clear
View Answer play_arrow
question_answer 291) Which of the following gland is often referred in relation with AIDS?
A)
Thyroid
done
clear
B)
Thymus
done
clear
C)
Adrenal
done
clear
D)
Pancreas
done
clear
View Answer play_arrow
question_answer 292) Ball and socket joints can be seen in
A)
wrist
done
clear
B)
fingers
done
clear
C)
neck
done
clear
D)
shoulders
done
clear
View Answer play_arrow
question_answer 293) Most simple amino acid is
A)
lysine
done
clear
B)
glycine
done
clear
C)
aspartic acid
done
clear
D)
nucleic acid
done
clear
View Answer play_arrow
question_answer 294) What is the correct sequence of thickness of muscles layers in the stomach of human beings?
A)
Circular\[\to \]Oblique\[\to \]Longitudinal
done
clear
B)
Oblique\[\to \]Longitudinal\[\to \]Circular
done
clear
C)
Longitudinal\[\to \]Circular\[\to \]Oblique
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 295) Blind sac body plan is shown by
A)
roundworms
done
clear
B)
annelids
done
clear
C)
coelenterates
done
clear
D)
arthropods
done
clear
View Answer play_arrow
question_answer 296) The typhlosole in earthworm is related with
A)
excretion
done
clear
B)
absorption
done
clear
C)
respiration
done
clear
D)
reproduction
done
clear
View Answer play_arrow
question_answer 297) Kaziranga wild life sanctuary is famous for
A)
tiger
done
clear
B)
musk deer
done
clear
C)
elephant
done
clear
D)
rhino
done
clear
View Answer play_arrow
question_answer 298) The famous Chipko Movement was started by
A)
Bahuguna
done
clear
B)
Rajeev Gandhi
done
clear
C)
Indira Gandhi
done
clear
D)
Salim Ali
done
clear
View Answer play_arrow
question_answer 299) Nif genes occur in
A)
Rhizobium
done
clear
B)
Aspergillus
done
clear
C)
Penicillium
done
clear
D)
Streptococcus
done
clear
View Answer play_arrow
question_answer 300) Kochs postulates are not applicable to
A)
TB
done
clear
B)
leprosy
done
clear
C)
cholera
done
clear
D)
diphtheria
done
clear
View Answer play_arrow
 Two equal metal balls are charged to 10 and -20 units of electricity. Then they are brought in contact with each other and then again separated to the original distance. The ratio of magnitudes of the force between the two balls before and after contact is
Two equal metal balls are charged to 10 and -20 units of electricity. Then they are brought in contact with each other and then again separated to the original distance. The ratio of magnitudes of the force between the two balls before and after contact is
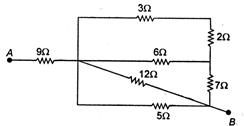
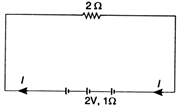
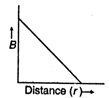
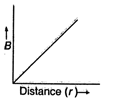
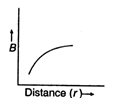
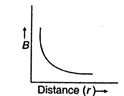
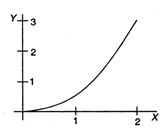
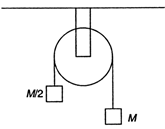
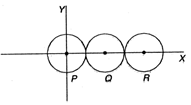
 What is the capacitance between P and Q?
What is the capacitance between P and Q?
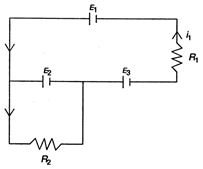
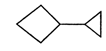
 Brown ring is made for
Brown ring is made for
