question_answer 1)
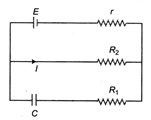
The charge on the capacitor of capacitance C shown in the figure below will be
A)
\[C\,E\]
done
clear
B)
\[\frac{C\,E\,{{R}_{1}}}{{{R}_{1}}+r}\]
done
clear
C)
\[\frac{C\,E\,{{R}_{2}}}{{{R}_{2}}+r}\]
done
clear
D)
\[\frac{C\,E\,{{R}_{1}}}{{{R}_{2}}+r}\]
done
clear
View Answer play_arrow
question_answer 2)
The resistance across A and B in the figure below will be
A)
3R
done
clear
B)
R
done
clear
C)
\[\frac{R}{3}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
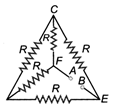
question_answer 3)
Five equal resistance, each of resistance R are connected as shown in figure below. A battery of V volt is connected between A and B. The current flowing in FC will be
A)
\[\frac{3V}{R}\]
done
clear
B)
\[\frac{V}{R}\]
done
clear
C)
\[\frac{V}{2R}\]
done
clear
D)
\[\frac{2V}{R}\]
done
clear
View Answer play_arrow
question_answer 4) Two cells with the same emf E and different internal resistances \[{{r}_{1}}\] and \[{{r}_{2}}\] are connected in series to an external resistance R. The value of R so that the potential difference across the first cell be zero, is
A)
\[\sqrt{{{r}_{1}}{{r}_{2}}}\]
done
clear
B)
\[{{r}_{1}}+{{r}_{2}}\]
done
clear
C)
\[{{r}_{1}}-{{r}_{2}}\]
done
clear
D)
\[\frac{{{r}_{1}}+{{r}_{2}}}{2}\]
done
clear
View Answer play_arrow
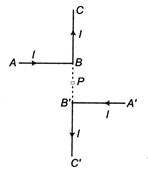
question_answer 5)
Current through ABC and \[ABC\]is\[I\]. What is the magnetic field at P? BP = PB = r (Here C B PBC are collinear)
A)
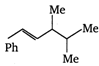
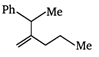
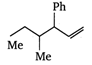
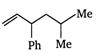
\[B=\frac{1}{4\pi }\frac{2I}{r}\]
done
clear
B)
\[B=\frac{{{\mu }_{0}}}{4\pi }\left( \frac{2I}{r} \right)\]
done
clear
C)
\[B=\frac{{{\mu }_{0}}}{4\pi }\left( \frac{I}{r} \right)\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 6) The magnetic field at the point of intersection of diagonals of a square wire loop of side L carrying a current \[I\] is
A)
\[\frac{{{\mu }_{0}}I}{\pi L}\]
done
clear
B)
\[\frac{2{{\mu }_{0}}I}{\pi L}\]
done
clear
C)
\[\frac{\sqrt{2}{{\mu }_{0}}I}{\pi L}\]
done
clear
D)
\[\frac{2\sqrt{2}{{\mu }_{0}}I}{\pi L}\]
done
clear
View Answer play_arrow
question_answer 7) In an inelastic collision an electron excites a hydrogen atom from its ground state to a M-shell state. A second electron collides instantaneously with the excited hydrogen atom in the M-shell state and ionizes it. At least how much energy the second electron transfers to the atom in the M-shell state?
A)
+3.4eV
done
clear
B)
+1.51eV
done
clear
C)
-3.4 eV
done
clear
D)
-1.51 eV
done
clear
View Answer play_arrow
question_answer 8) A radioactive nucleus of mass number A, initially at rest, emits an \[\text{ }\!\!\alpha\!\!\text{ -}\]particle with a speed v. The recoil speed of the daughter nucleus will be
A)
\[\frac{2v}{A-4}\]
done
clear
B)
\[\frac{2v}{A+4}\]
done
clear
C)
\[\frac{4v}{A-4}\]
done
clear
D)
\[\frac{4v}{A+4}\]
done
clear
View Answer play_arrow
question_answer 9) In the nuclear reaction \[_{7}^{14}N+X\xrightarrow{{}}_{6}^{14}C+_{1}^{1}H,\]the X will be
A)
\[_{-1}^{0}e\]
done
clear
B)
\[_{1}^{1}H\]
done
clear
C)
\[_{1}^{2}H\]
done
clear
D)
\[_{0}^{1}n\]
done
clear
View Answer play_arrow
question_answer 10)
Which type of gate the following truth table represents? Input Output A B Q 0 0 1 0 1 1 1 0 1 1 1 0
A)
NOT
done
clear
B)
AND
done
clear
C)
OR
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 11) Given \[\vec{A}=2\hat{i}+3\hat{j}\]and \[\vec{B}=\hat{i}+\hat{j}.\]The component of vector \[\vec{A}\] along vector \[\vec{B}\] is
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\frac{3}{\sqrt{2}}\]
done
clear
C)
\[\frac{5}{\sqrt{2}}\]
done
clear
D)
\[\frac{7}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 12) A cubical vessel of height 1 m is full of water. What is the amount of work done in pumping water out of the vessel? (Takeg \[g=10\,m/{{s}^{2}}\])
A)
1250 J
done
clear
B)
5000 J
done
clear
C)
1000 J
done
clear
D)
2500 J
done
clear
View Answer play_arrow
question_answer 13) A stone of relative density K is released from rest on the surface of a lake. If viscous effects are ignored, the stone sinks in water with an acceleration of
A)
\[g(1-K)\]
done
clear
B)
\[g(1+K)\]
done
clear
C)
\[g\left( 1-\frac{1}{K} \right)\]
done
clear
D)
\[g\left( 1+\frac{1}{K} \right)\]
done
clear
View Answer play_arrow
question_answer 14) If a person can throw a stone to maximum height of metre vertically, then the maximum distance through which it can be thrown horizontally by the same person is
A)
\[\frac{h}{2}\]
done
clear
B)
\[h\]
done
clear
C)
\[2h\]
done
clear
D)
\[3h\]
done
clear
View Answer play_arrow
question_answer 15) A body of mass 6 kg is acted upon by a force which causes a displacement in it given by \[x=\frac{{{t}^{2}}}{4}\] metre, where t is the time in second. 4 The work done by the force in 2 s is
A)
12J
done
clear
B)
9J
done
clear
C)
6J
done
clear
D)
3J
done
clear
View Answer play_arrow
question_answer 16) A box is moved along a straight line by a machine delivering constant power. The distance moved by the body in time t is proportional to
A)
\[{{t}^{\frac{1}{2}}}\]
done
clear
B)
\[{{t}^{\frac{3}{4}}}\]
done
clear
C)
\[{{t}^{\frac{3}{2}}}\]
done
clear
D)
\[{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 17) A particle is moving with a constant speed v in a circle. What is the magnitude of average velocity after half rotation?
A)
2v
done
clear
B)
\[2\frac{v}{\pi }\]
done
clear
C)
\[\frac{v}{2}\]
done
clear
D)
\[\frac{v}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 18) A cricket ball of mass 0.25 kg with speed 10 m/s collides with a bat and returns with same speed within 0.01 s. The force acted on bat is
A)
25 N
done
clear
B)
50 N
done
clear
C)
250 N
done
clear
D)
500 N
done
clear
View Answer play_arrow
question_answer 19) If the earth were to suddenly contract to \[\frac{\text{1}}{\text{n}}\text{th}\] of its present radius without any change in its mass, the duration of the new day will be nearly
A)
\[24/n\,h\]
done
clear
B)
\[24n\,h\]
done
clear
C)
\[24/{{n}^{2}}\,h\]
done
clear
D)
\[24\,{{n}^{2}}\,h\]
done
clear
View Answer play_arrow
question_answer 20) If g is the acceleration due to gravity on the surface of the earth, the gain in potential energy of an object of mass m raised from the earths surface to a height equal to the radius R of the earth is
A)
\[\frac{mgR}{4}\]
done
clear
B)
\[\frac{mgR}{2}\]
done
clear
C)
\[mgR\]
done
clear
D)
\[2mgR\]
done
clear
View Answer play_arrow
question_answer 21) A material has Poissons ratio 0.50. If a uniform rod of it suffers a longitudinal strain of \[2\times {{10}^{-3}},\] then the percentage change in volume is
A)
0.6
done
clear
B)
0.4
done
clear
C)
0.2
done
clear
D)
zero
done
clear
View Answer play_arrow
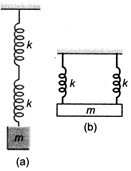
question_answer 22)
Two identical springs are connected to mass m as shown (\[k=\]spring constant). If the period of the configuration in is 2s, the period of the configuration in is
A)
\[\sqrt{2}\,s\]
done
clear
B)
1s
done
clear
C)
\[\frac{1}{\sqrt{2}}s\]
done
clear
D)
\[2\sqrt{2}\,s\]
done
clear
View Answer play_arrow
question_answer 23) An object weights \[{{m}_{1}}\]in a liquid of density \[{{d}_{1}}\]and that in liquid of density \[{{d}_{2}}\]is \[{{m}_{2}}.\]density d of the object is
A)
\[\frac{{{m}_{2}}{{d}_{2}}-{{m}_{1}}{{d}_{1}}}{{{m}_{2}}-{{m}_{1}}}\]
done
clear
B)
\[\frac{{{m}_{1}}{{d}_{1}}-{{m}_{2}}{{d}_{2}}}{{{m}_{2}}-{{m}_{1}}}\]
done
clear
C)
\[\frac{{{m}_{2}}{{d}_{1}}-{{m}_{1}}{{d}_{2}}}{{{m}_{1}}-{{m}_{2}}}\]
done
clear
D)
\[\frac{{{m}_{1}}{{d}_{2}}-{{m}_{2}}{{d}_{1}}}{{{m}_{1}}-{{m}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 24) A body floats in water with 40% of its volume outside water. When the same body floats in an oil. 60% of its volume remains outside oil. The relative density of oil is
A)
0.9
done
clear
B)
1.0
done
clear
C)
1.2
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 25) Two soap bubbles of radii \[x\] and \[y\] coalesce to constitute a bubble of radius z. Then z is equal to___
A)
\[\sqrt{{{x}^{2}}+{{y}^{2}}}\]
done
clear
B)
\[\sqrt{x+y}\]
done
clear
C)
\[x+y\]
done
clear
D)
\[\frac{x+y}{2}\]
done
clear
View Answer play_arrow
question_answer 26) A particle of mass m is located in a one dimensional potential field where potential energy is given by \[V(x)=A(1-\cos \,px),\]where A and p are constants. The period of small oscillations of the particle is
A)
\[2\pi \sqrt{\frac{m}{(Ap)}}\]
done
clear
B)
\[2\pi \sqrt{\frac{m}{(A{{p}^{2}})}}\]
done
clear
C)
\[2\pi \sqrt{\frac{m}{A}}\]
done
clear
D)
\[\frac{1}{2\pi }\sqrt{\frac{Ap}{m}}\]
done
clear
View Answer play_arrow
question_answer 27) The period of oscillation of a simple pendulum of length \[l\] suspended from the roof of a vehicle, which moves without friction down an inclined plane of inclination \[\alpha ,\]is given by
A)
\[2\pi \sqrt{\frac{l}{g\,\cos \,\alpha }}\]
done
clear
B)
\[2\pi \sqrt{\frac{l}{g\,\sin \,\alpha }}\]
done
clear
C)
\[2\pi \sqrt{\frac{l}{g\,}}\]
done
clear
D)
\[2\pi \sqrt{\frac{l}{g\,\tan \,\alpha }}\]
done
clear
View Answer play_arrow
question_answer 28) In Youngs double slit experiment the two slits are d distance apart. Interference pattern is observed on a screen at a distance D from the slits. A dark fringe is observed on the screen directly opposite to one of the slits. The wavelength of light is
A)
\[\frac{{{D}^{2}}}{2d}\]
done
clear
B)
\[\frac{{{d}^{2}}}{2D}\]
done
clear
C)
\[\frac{{{D}^{2}}}{d}\]
done
clear
D)
\[\frac{{{d}^{2}}}{D}\]
done
clear
View Answer play_arrow
question_answer 29) A plane progressive wave is given by \[y=2\cos 6.284(330t-x).\]What is period of the wave?
A)
\[\frac{1}{330}s\]
done
clear
B)
\[2\pi \times 330\,s\]
done
clear
C)
\[{{(2\pi \times 330)}^{-1}}s\]
done
clear
D)
\[\frac{6.284}{330}s\]
done
clear
View Answer play_arrow
question_answer 30) The displacement of a particle is SHM varies according to the relation \[x=4(cos\pi t+sin\pi t).\]The amplitude of the particle is
A)
\[-4\]
done
clear
B)
4
done
clear
C)
\[4\sqrt{2}\]
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 31) Two temperature scales A and B are related by \[\frac{A-42}{110}=\frac{B-72}{220}.\] which temperature two scales have the same reading?
A)
\[-{{42}^{o}}\]
done
clear
B)
\[-{{72}^{o}}\]
done
clear
C)
\[+\,{{12}^{o}}\]
done
clear
D)
\[-{{40}^{o}}\]
done
clear
View Answer play_arrow
question_answer 32) An ideal gas is compressed isothermally until its pressure is doubled and then allowed to expand adiabatically to regain its original volume (\[\gamma =1.4\]and\[{{2}^{-14}}=0.38\]). The ratio of the final to initial pressure is
A)
0.76 : 1
done
clear
B)
1 : 1
done
clear
C)
0.66 : 1
done
clear
D)
0.86 : 1
done
clear
View Answer play_arrow
question_answer 33) Air inside a closed container is saturated with water vapour. The air pressure is p and the saturated vapour pressure of water is \[\bar{P}\].If the mixture is compressed to one-half of its volume by maintaining temperature constant, the pressure becomes
A)
\[2(p+\bar{p})\]
done
clear
B)
\[2p+\bar{p}\]
done
clear
C)
\[(p+\bar{p})/2\]
done
clear
D)
\[p+2\bar{p}\]
done
clear
View Answer play_arrow
question_answer 34) 1\[1.56\times {{10}^{5}}\,J\] of heat is conducted through a \[\text{2}\,{{\text{m}}^{\text{2}}}\]wall of 12 cm thick in 1h. Temperature difference between the two sides of the wall is \[\text{20}{{\,}^{\text{o}}}\text{C}\text{.}\] The thermal conductivity of the material of the wall is (in \[\text{W}\,{{\text{m}}^{-1}}{{K}^{-1}}\])
A)
0.11
done
clear
B)
0.13
done
clear
C)
0.15
done
clear
D)
1.2
done
clear
View Answer play_arrow
question_answer 35) A diver at a depth of 12 m in water \[\left( \mu =\frac{4}{3} \right)\] sees the sky in a cone of semivertical angle
A)
\[{{\sin }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
D)
\[\text{9}{{\text{0}}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 36) Two thin lenses of focal lengths 20 cm and 25 cm are placed in contact. The effective power of the combination is
A)
9D
done
clear
B)
2D
done
clear
C)
3D
done
clear
D)
7D
done
clear
View Answer play_arrow
question_answer 37) A convex lens of focal length 30 cm produces 5 times magnified real image of an object. What is the object distance?
A)
36 cm
done
clear
B)
25 cm
done
clear
C)
30 cm
done
clear
D)
150 cm
done
clear
View Answer play_arrow
question_answer 38) If the focal length of the eye-piece of a telescope is doubled, its magnifying power (m) will be
A)
\[2\,m\]
done
clear
B)
\[3\,m\]
done
clear
C)
\[\frac{m}{2}\]
done
clear
D)
\[4m\]
done
clear
View Answer play_arrow
question_answer 39) A plano-concave lens is made of glass of refractive index 1.5 and the radius of curvature of its curved face is 100 cm. What is the power of the lens?
A)
\[+\,0.5\,D\]
done
clear
B)
\[-0.5\,D\]
done
clear
C)
\[-2\,D\]
done
clear
D)
\[+\,2\,D\]
done
clear
View Answer play_arrow
question_answer 40) Four charges equal to -Q are placed at the four comers of a square and a charge q is at its centre. If the system is in equilibrium, the value of q is
A)
\[\frac{-Q}{4}(1+2\sqrt{2})\]
done
clear
B)
\[\frac{Q}{4}(1+2\sqrt{2})\]
done
clear
C)
\[\frac{-Q}{2}(1+2\sqrt{2})\]
done
clear
D)
\[\frac{Q}{2}(1+2\sqrt{2})\]
done
clear
View Answer play_arrow
question_answer 41) Two aromatic compounds having formula \[{{C}_{7}}{{H}_{8}}O\]which are easily identifiable by \[FeC{{l}_{3}}\]solution test (violet colouration) are
A)
o-cresol and benzyl alcohol
done
clear
B)
m-cresol and p-cresol
done
clear
C)
o-cresol and p-cresol
done
clear
D)
methyl phenyl ether and benzyl alcohol
done
clear
View Answer play_arrow
question_answer 42) The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is
A)
\[{{3}^{o}}<{{2}^{o}}<{{1}^{o}}\]
done
clear
B)
\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\]
done
clear
C)
\[{{3}^{o}}<{{2}^{o}}>{{1}^{o}}\]
done
clear
D)
\[{{3}^{o}}>{{2}^{o}}<{{1}^{o}}\]
done
clear
View Answer play_arrow
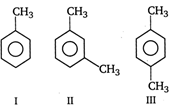
question_answer 43)
The ease of nitration of the following three hydrocarbons follows the order
A)
II = III = I
done
clear
B)
II > III > I
done
clear
C)
III > II > I
done
clear
D)
I = III > II
done
clear
View Answer play_arrow
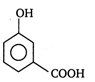
question_answer 44) The correct order of decreasing acidity of nitrophenols will be
A)
m- nitrophenol>p-nitrophenol>o-nitrophenol
done
clear
B)
o-nitrophenol >m-nitrophenol> p-nitrophenol
done
clear
C)
p-nitrophenol>m-mtrophenol> o-nitrophenol
done
clear
D)
p-nitrophenol > o-nitrophenol > m-nitrophenol
done
clear
View Answer play_arrow
question_answer 45) Among the alkenes which one produces tertiary butyl alcohol on acid hydration?
A)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}CH=CHC{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 46) Which of the following compounds has maximum volatility?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 47) Which one of the following will show optical isomerism?
A)
\[HO-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C-C{{O}_{2}}H}}}\,}}}\,\]
done
clear
B)
\[{{H}_{3}}C-\underset{OH}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C-C{{O}_{2}}H}}}\,}}}\,\]
done
clear
C)
\[{{H}_{3}}C-\underset{H}{\overset{C\,{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C-C{{O}_{2}}H}}}\,}}}\,\]
done
clear
D)
\[{{H}_{3}}C-\underset{Cl}{\overset{C\,{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C-C{{O}_{2}}H}}}\,}}}\,\]
done
clear
View Answer play_arrow
question_answer 48) The pH of an aqueous solution of \[C{{H}_{3}}COONa\]of concentration C(M) is given by
A)
\[7-\frac{1}{2}p{{K}_{a}}-\frac{1}{2}\log \,C\]
done
clear
B)
\[\frac{1}{2}p{{K}_{w}}+\frac{1}{2}p{{K}_{b}}+\frac{1}{2}\log \,C\]
done
clear
C)
\[\frac{1}{2}p{{K}_{w}}-\frac{1}{2}p{{K}_{b}}-\frac{1}{2}\log \,C\]
done
clear
D)
\[\frac{1}{2}p{{K}_{w}}+\frac{1}{2}p{{K}_{a}}+\frac{1}{2}\log \,C\]
done
clear
View Answer play_arrow
question_answer 49) The standard reduction potential \[{{\text{E}}^{\text{o}}}\]for half- reaction are \[Zn\to Z{{n}^{2+}}+2{{e}^{-}};{{E}^{o}}=+0.76\,V\] \[Fe\to F{{e}^{2+}}+2{{e}^{-}};{{E}^{o}}=+\,0.41\,V\] The EMF of the cell reaction \[F{{e}^{2+}}+Zn\to Z{{n}^{2+}}+Fe\]is
A)
\[-0.35\,V\]
done
clear
B)
\[+\,0.35\text{ }V\]
done
clear
C)
\[+1.17\,V\]
done
clear
D)
\[-1.17\,V\]
done
clear
View Answer play_arrow
question_answer 50) If the equilibrium constants of the following equilibria, \[S{{O}_{2}}+\frac{1}{2}{{O}_{2}}\to S{{O}_{3}}\]and \[2S{{O}_{3}}\to 2S{{O}_{2}}+{{O}_{2}}\] are given by \[{{K}_{1}}\] and \[{{K}_{2}}\] respectively, which of the following relations is correct?
A)
\[{{K}_{2}}={{\left( \frac{1}{{{K}_{1}}} \right)}^{2}}\]
done
clear
B)
\[{{K}_{1}}={{\left( \frac{1}{{{K}_{2}}} \right)}^{3}}\]
done
clear
C)
\[{{K}_{2}}=\left( \frac{1}{{{K}_{1}}} \right)\]
done
clear
D)
\[{{K}_{2}}={{({{K}_{1}})}^{2}}\]
done
clear
View Answer play_arrow
question_answer 51) The energy of an electron in first Bohr orbit of H-atom is -13.6 eV. The possible energy value of electron in the excited state of \[\text{L}{{\text{i}}^{\text{2+}}}\] is
A)
-122.4 eV
done
clear
B)
30.6 eV
done
clear
C)
-30.6 eV
done
clear
D)
13.6 eV
done
clear
View Answer play_arrow
question_answer 52) The amount of the heat released when 20 mL 0.5 M NaOH is mixed with 100 mL M HCl is \[x\,kJ.\] The heat of neutralization is
A)
\[-100\,x\,\text{kJ/mol}\]
done
clear
B)
\[-50\,x\,\text{kJ/mol}\]
done
clear
C)
\[+\,100\,x\,\text{kJ/mol}\]
done
clear
D)
\[+50\,x\,\text{kJ/mol}\]
done
clear
View Answer play_arrow
question_answer 53) Which one of the following has the lowest ionisation energy?
A)
\[1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{6}}\]
done
clear
B)
\[1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{6}},3{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}},\,2{{s}^{2}},\,2{{p}^{5}}\]
done
clear
D)
\[1s,\,2{{s}^{2}},\,2{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 54) The ozone layer forms naturally by
A)
the interaction of CFC with oxygen
done
clear
B)
the interaction of UV radiation with oxygen
done
clear
C)
the interaction of IR radiation with oxygen
done
clear
D)
the interaction of oxygen and water vapour
done
clear
View Answer play_arrow
question_answer 55) 2 g of metal carbonate is neutralized completely by 100 mL of 0.1 N HCl. The equivalent weight of metal carbonate is
A)
50
done
clear
B)
100
done
clear
C)
150
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 56) Which one of the following is not true at room temperature and pressure?
A)
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\] is white solid
done
clear
B)
\[\text{S}{{\text{O}}_{2}}\]is a colourless gas
done
clear
C)
\[\text{S}{{\text{O}}_{3}}\]is a colourless gas
done
clear
D)
\[\text{N}{{\text{O}}_{2}}\]is a brown gas
done
clear
View Answer play_arrow
question_answer 57) An electric current is passed through an aqueous solution of a mixture of alanine (isoelectric point 6.0), glutamic acid (3.2) and arginine (10.7) buffered at pH 6. What is the fate of the three acids?
A)
Glutamic acid migrates to anode at pH 6. Arginine is present as a cation and migrates to the cathode. Alanine in a dipolar ion remains uniformly distributed in solution
done
clear
B)
Glutamic acid migrates to cathode and others remain uniformly distributed in solution
done
clear
C)
All three remain uniformly distributed in solution
done
clear
D)
All three move to cathode
done
clear
View Answer play_arrow
question_answer 58) The representation of the ground state electronic configuration of He by box-diagram as \[\] is wrong because it violates
A)
Heisenbergs uncertainty principle
done
clear
B)
Bohrs quantization theory of angular momenta
done
clear
C)
Pauli exclusion principle
done
clear
D)
Hunds rule
done
clear
View Answer play_arrow
question_answer 59) The electronic transitions from \[n=2\] to \[n=1\]will produce shortest wavelength in (where n = principal quantum state)
A)
\[L{{i}^{2+}}\]
done
clear
B)
\[H{{e}^{+}}\]
done
clear
C)
H
done
clear
D)
\[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 60) In the following electron-dot structure, calculate the formal charge from left to right nitrogen atom; \[\underset{\centerdot \,\,\centerdot }{\overset{\centerdot \,\,\centerdot }{\mathop{N}}}\,=N=\underset{\centerdot \,\,\centerdot }{\overset{\centerdot \,\,\centerdot }{\mathop{N}}}\,\]
A)
\[-1,-\text{ }1,+\text{ }1\]
done
clear
B)
\[-1,+1,-1\]
done
clear
C)
\[+1,-\text{ }1,-1\]
done
clear
D)
\[+1,-\text{ }1,+1\]
done
clear
View Answer play_arrow
question_answer 61) If the molecular wt. of \[\text{N}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]and \[{{\text{I}}_{\text{2}}}\] are \[{{\text{M}}_{1}}\]and \[{{\text{M}}_{2}}\]respectively then what will be the equivalent weight of \[\text{N}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]and \[{{\text{I}}_{2}}\]in the following reaction? \[2{{S}_{2}}O_{3}^{2-}+{{I}_{2}}\xrightarrow{{}}{{S}_{4}}O_{6}^{2-}+2{{I}^{-}}\]
A)
\[{{M}_{1}},{{M}_{2}}\]
done
clear
B)
\[{{M}_{1}},{{M}_{2}}/2\]
done
clear
C)
\[2{{M}_{1}},{{M}_{2}}\]
done
clear
D)
\[{{M}_{1}}2{{M}_{2}}\]
done
clear
View Answer play_arrow
question_answer 62) A radioactive atom \[_{\text{Y}}^{\text{X}}\text{M}\]emits two \[\alpha \] particles and one P particle successively. The number of neutrons in the nucleus of the product will be
A)
\[X-4-Y\]
done
clear
B)
\[X-Y-5\]
done
clear
C)
\[X-Y-3\]
done
clear
D)
\[X-Y-6\]
done
clear
View Answer play_arrow
question_answer 63) An element belongs to group 15 and third period of the Periodic Table. Its electronic configuration will be
A)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{3}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Which one of the following is paramagnetic?
A)
\[{{N}_{2}}\]
done
clear
B)
NO
done
clear
C)
CO
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 65) Platinum, palladium and iridium are called noble metals because
A)
Alfred Nobel discovered them
done
clear
B)
they are shining lustrous and pleasing to look at
done
clear
C)
they are found in native state
done
clear
D)
they are inert towards many common reagents
done
clear
View Answer play_arrow
question_answer 66) Which one is not a constituent of nucleic acid?
A)
Uracil
done
clear
B)
Guanidine
done
clear
C)
Phosphoric acid
done
clear
D)
Ribose sugar
done
clear
View Answer play_arrow
question_answer 67) The \[s{{p}^{3}}{{d}^{2}}\]hybridisation of central atom of a molecule would lead to
A)
square planar geometry
done
clear
B)
tetrahedral geometry
done
clear
C)
trigonal bipyramidal geometry
done
clear
D)
octahedral geometry
done
clear
View Answer play_arrow
question_answer 68) In aqueous solution glucose remains as
A)
only in open chain form
done
clear
B)
only in pyranose form
done
clear
C)
only in furanose form
done
clear
D)
in all three forms in equilibrium
done
clear
View Answer play_arrow
question_answer 69) Which of the following is used to prepare \[\text{C}{{\text{l}}_{\text{2}}}\]gas at room temperature from concentrated HCl?
A)
\[\text{Mn}{{\text{O}}_{2}}\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[KMn{{O}_{4}}\]
done
clear
D)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) \[\text{N}{{\text{O}}_{\text{2}}}\]is not obtained on heating
A)
\[AgN{{O}_{3}}\]
done
clear
B)
\[KN{{O}_{3}}\]
done
clear
C)
\[Cu{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
\[Pb{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) The normality of 30 volume \[{{H}_{2}}{{O}_{2}}\]is
A)
2.678 N
done
clear
B)
5.336 N
done
clear
C)
8.034 N
done
clear
D)
6.685 N
done
clear
View Answer play_arrow
question_answer 72) Reaction of formaldehyde and ammonia gives
A)
hexamethylene tetramine
done
clear
B)
bake lite
done
clear
C)
urea
done
clear
D)
triethylene tetramine
done
clear
View Answer play_arrow
question_answer 73) A plot of In K against\[\frac{1}{T}\] (abscissa) is expected to be a straight line with intercept on ordinate axis equal to
A)
\[\frac{\Delta {{S}^{o}}}{2.303R}\]
done
clear
B)
\[\frac{\Delta {{S}^{o}}}{R}\]
done
clear
C)
\[-\frac{\Delta {{S}^{o}}}{R}\]
done
clear
D)
\[R\times \Delta {{S}^{o}}\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following represents the composition of camallite mineral?
A)
\[{{K}_{2}}O.A{{l}_{2}}{{O}_{3}}.6Si{{O}_{2}}\]
done
clear
B)
\[KN{{O}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}.MgS{{O}_{4}}.MgC{{l}_{2}}.6{{H}_{2}}O\]
done
clear
D)
\[KCl.MgC{{l}_{2}}.6{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 75) The solubility of \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\]in water is y mol/L. Its solubility product is
A)
\[6{{y}^{4}}\]
done
clear
B)
\[36{{y}^{4}}\]
done
clear
C)
\[64{{y}^{5}}\]
done
clear
D)
\[108{{y}^{5}}\]
done
clear
View Answer play_arrow
question_answer 76) Paracetamol is
A)
methyl salicylate
done
clear
B)
phenyl salicylate
done
clear
C)
N-acetyl p-amino phenol
done
clear
D)
acetyl salicylic acid
done
clear
View Answer play_arrow
question_answer 77) Anhydrous ferric chloride is prepared by
A)
dissoving \[Fe{{(OH)}_{3}}\]in concentrated \[HCl\]
done
clear
B)
dissolving \[Fe{{(OH)}_{3}}\]in dilute HCl
done
clear
C)
passing dry HCl over heated iron scrap
done
clear
D)
passing dry \[\text{C}{{\text{l}}_{\text{2}}}\]gas over heated iron scrap
done
clear
View Answer play_arrow
question_answer 78) Which one of the following is 5-butyl phenyl vinyl methane?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 79) Hybridisation of \[{{C}_{2}}\] and \[{{C}_{3}}\] of \[{{H}_{3}}C-CH=CH-C{{H}_{3}}\]are
A)
\[sp,s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}},sp\]
done
clear
C)
\[s{{p}^{2}},s{{p}^{2}}\]
done
clear
D)
\[sp,sp\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following compounds is not formed in iodoform reaction of acetone?
A)
\[C{{H}_{3}}COC{{H}_{2}}I\]
done
clear
B)
\[IC{{H}_{2}}COC{{H}_{2}}I\]
done
clear
C)
\[C{{H}_{3}}COCH{{I}_{2}}\]
done
clear
D)
\[C{{H}_{3}}COC{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) Glucose and amino acids are reabsorbed in the
A)
proximal tubule
done
clear
B)
distal tubule
done
clear
C)
collecting duct
done
clear
D)
loop of Henie
done
clear
View Answer play_arrow
question_answer 82) The amount of CSF in the cranial cavity
A)
500 mL
done
clear
B)
140 mL
done
clear
C)
1 litre
done
clear
D)
1.5 mL
done
clear
View Answer play_arrow
question_answer 83) Which one is imino add?
A)
Pepsin
done
clear
B)
Proline
done
clear
C)
Cysteine
done
clear
D)
Renin
done
clear
View Answer play_arrow
question_answer 84) The main difference between Gram positive and Gram negative bacteria is
A)
cell membrane
done
clear
B)
cell wall
done
clear
C)
ribosome
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 85) ACTH is secreted from
A)
adrenal cortex
done
clear
B)
pituitary
done
clear
C)
adrenal medulla
done
clear
D)
thyroid
done
clear
View Answer play_arrow
question_answer 86) Which of the following is the correct pathway for propagation of cardiac impulse?
A)
SA node\[\to \]AV node\[\to \]Bundle of His\[\to \] Purkinje fibres
done
clear
B)
AV node \[\to \] Bundle of His \[\to \] SA node \[\to \] Purkinje fibres
done
clear
C)
SA node \[\to \] Purkinje fibres \[\to \] AV node \[\to \] Bundle of His
done
clear
D)
Purkinje fibres\[\to \]AV node\[\to \]SA node\[\to \] Bundle of His
done
clear
View Answer play_arrow
question_answer 87) Inner surface of the bronchi, bronchioles and fallopian tubes are lined by
A)
cubical epithelium
done
clear
B)
columnar epithelium
done
clear
C)
squamous epithelium
done
clear
D)
ciliated epithelium
done
clear
View Answer play_arrow
question_answer 88) Electric potential of the brain is recorded by
A)
CT scan
done
clear
B)
Sphygmomanometer
done
clear
C)
ECG
done
clear
D)
EEG
done
clear
View Answer play_arrow
question_answer 89) Which of the following is related to humoral immunity?
A)
T-lymphocyte
done
clear
B)
B-lymphocyte
done
clear
C)
I-lymphocyte
done
clear
D)
P-lymphocyte
done
clear
View Answer play_arrow
question_answer 90) Fertilization occur in
A)
uterus
done
clear
B)
ureter
done
clear
C)
vagina
done
clear
D)
fallopian tube
done
clear
View Answer play_arrow
question_answer 91) The gastrin is secreted from
A)
intestine
done
clear
B)
stomach
done
clear
C)
pancreas
done
clear
D)
rectum
done
clear
View Answer play_arrow
question_answer 92) The cause of cretinism is
A)
hypothyroidism
done
clear
B)
hypoparathyroidism
done
clear
C)
hyperthyroidism
done
clear
D)
hyperparathyroidism
done
clear
View Answer play_arrow
question_answer 93) Which of the following is a minerelocorticoid?
A)
Testosterone
done
clear
B)
Progesterone
done
clear
C)
Adrenalin
done
clear
D)
Aldosterone
done
clear
View Answer play_arrow
question_answer 94) The part of the brain where the centre for hunger and thirst is located is
A)
cerebrum
done
clear
B)
hypothalamus
done
clear
C)
cerebellum
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 95) The reflex arc, which is made of two neurons is known as
A)
monosynaptic reflex arc
done
clear
B)
disynaptic reflex arc
done
clear
C)
polysynaptic reflex arc
done
clear
D)
asynaptic reflex arc
done
clear
View Answer play_arrow
question_answer 96) The lactase hydrolyses lactose into
A)
glucose
done
clear
B)
glucose and galactose
done
clear
C)
fructose
done
clear
D)
glucose and fructose
done
clear
View Answer play_arrow
question_answer 97) In 24 hours, total glomerular filtrate formed in human kidney is
A)
1.7 litres
done
clear
B)
7 litres
done
clear
C)
17 litres
done
clear
D)
170 litres
done
clear
View Answer play_arrow
question_answer 98) When the oxygen supply to the tissue in inadequate, the condition is
A)
dyspnea
done
clear
B)
hypoxia
done
clear
C)
asphyxia
done
clear
D)
apnea
done
clear
View Answer play_arrow
question_answer 99) Which one of the following is not a second messenger in hormone action?
A)
Calcium
done
clear
B)
Sodium
done
clear
C)
c AMP
done
clear
D)
c GMP
done
clear
View Answer play_arrow
question_answer 100) The name of the pace maker of the heart is
A)
lymph node
done
clear
B)
SA node
done
clear
C)
juxtaglomerular apparatus
done
clear
D)
semilunar valve
done
clear
View Answer play_arrow
question_answer 101) What is a genophore?
A)
DNA in prokaryotes
done
clear
B)
DNA and RNA in prokaryotes
done
clear
C)
DNA and protein in prokaryotes
done
clear
D)
RNA in prokaryotes
done
clear
View Answer play_arrow
question_answer 102) Example of a typical homopolysaccharide is
A)
lignin
done
clear
B)
suberin
done
clear
C)
inulin
done
clear
D)
starch
done
clear
View Answer play_arrow
question_answer 103) Who wrote the famous book Origin of Species?
A)
Lamarck
done
clear
B)
Darwin
done
clear
C)
de Vries
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 104) Polyploid derived from two different species is called
A)
autopolyploid
done
clear
B)
triploid
done
clear
C)
allopolyploid
done
clear
D)
monoploid
done
clear
View Answer play_arrow
question_answer 105) Electrons used in electron microscope are of the wavelength
A)
0.05\[Ao\]
done
clear
B)
0.15\[Ao\]
done
clear
C)
0.25\[Ao\]
done
clear
D)
0.30\[Ao\]
done
clear
View Answer play_arrow
question_answer 106) Biolistic technique is used in
A)
tissue culture process
done
clear
B)
gene transfer process
done
clear
C)
hybridization process
done
clear
D)
germplasm conservation process
done
clear
View Answer play_arrow
question_answer 107) Example of water soluble plant pigment is
A)
chlorophyll- a
done
clear
B)
chlorophyll- b
done
clear
C)
anthocyanin
done
clear
D)
xanthophylls
done
clear
View Answer play_arrow
question_answer 108) Structural element of chromatin is
A)
histone
done
clear
B)
acid protein and DNA
done
clear
C)
nuclear matrix
done
clear
D)
nucleosomes
done
clear
View Answer play_arrow
question_answer 109) Inulin is a polymer of
A)
glucose
done
clear
B)
galactose
done
clear
C)
fructose
done
clear
D)
arabinose
done
clear
View Answer play_arrow
question_answer 110) Mannitol is
A)
amino acid
done
clear
B)
amino alcohol
done
clear
C)
sugar alcohol
done
clear
D)
sugar acid
done
clear
View Answer play_arrow
question_answer 111) A flower which can be divided into two equal halves by only one plane is
A)
zygomorphic
done
clear
B)
actinomorphic
done
clear
C)
regular
done
clear
D)
perfect
done
clear
View Answer play_arrow
question_answer 112) Pieces of plant tissue used in culture is called
A)
explant
done
clear
B)
domaclone
done
clear
C)
inoculant
done
clear
D)
clone
done
clear
View Answer play_arrow
question_answer 113) VAM is
A)
symbiotic bacteria
done
clear
B)
saprophytic bacteria
done
clear
C)
saprophytic fungi
done
clear
D)
symbiotic fungi
done
clear
View Answer play_arrow
question_answer 114) Ovule integument gets transformed into
A)
seed
done
clear
B)
fruit wall
done
clear
C)
seed coat
done
clear
D)
cotyledons
done
clear
View Answer play_arrow
question_answer 115) Acid rain is caused by
A)
\[N{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\] and \[N{{O}_{2}}\]
done
clear
C)
\[S{{O}_{3}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 116) Which one of the following bacterium is used for production of transgenic plants
A)
Escherichia coli
done
clear
B)
Bacillus thuringiensis
done
clear
C)
Staphylococcus aureus
done
clear
D)
Agrobacterium tumefadens
done
clear
View Answer play_arrow
question_answer 117) A plant cell becomes turgid due to
A)
plasmolysis
done
clear
B)
exosmosis
done
clear
C)
endosmosis
done
clear
D)
electrolysis
done
clear
View Answer play_arrow
question_answer 118) Restriction enzymes are used to cut
A)
single stranded RNA
done
clear
B)
double stranded DNA
done
clear
C)
single stranded DNA
done
clear
D)
double stranded RNA
done
clear
View Answer play_arrow
question_answer 119) Spindle fibre is made up of
A)
humulin
done
clear
B)
intermediate filament
done
clear
C)
flagellin
done
clear
D)
tubulin
done
clear
View Answer play_arrow
question_answer 120) Edible part of mushroom is
A)
basidiocarp
done
clear
B)
primary mycelium
done
clear
C)
fungal hyphae
done
clear
D)
basidiospores
done
clear
View Answer play_arrow
question_answer 121) Calcium level decreases in the blood due to hyposecretion of
A)
parathyroid hormone
done
clear
B)
calcitonin
done
clear
C)
thyroxine
done
clear
D)
adrenaline
done
clear
View Answer play_arrow
question_answer 122) Kupffers cells are
A)
phagocytic
done
clear
B)
actin
done
clear
C)
myosin
done
clear
D)
fibrin
done
clear
View Answer play_arrow
question_answer 123) Which centre is stimulated during increase in body temperature?
A)
Anterior hypothalamus
done
clear
B)
Posterior hypothalamus
done
clear
C)
Limbic system
done
clear
D)
Red nucleus
done
clear
View Answer play_arrow
question_answer 124) Name the following having oxygen storing capacity
A)
myoglobin
done
clear
B)
prophase- II
done
clear
C)
anaphase- I
done
clear
D)
metaphase- II
done
clear
View Answer play_arrow
question_answer 125) Longest phase of meiosis
A)
prophase- I
done
clear
B)
prophase- II
done
clear
C)
anaphase- I
done
clear
D)
metaphase- II
done
clear
View Answer play_arrow
question_answer 126) Tetany is caused by
A)
hyperparathyroidism
done
clear
B)
hypoparathyroidism
done
clear
C)
hyperthyroidism
done
clear
D)
hypothyroidism
done
clear
View Answer play_arrow
question_answer 127) Which the following is a gastrointestme hormone?
A)
Prolactin
done
clear
B)
Enterogastrone
done
clear
C)
GH
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 128) Name the hormone that has no role in menstruation
A)
LH
done
clear
B)
FSH
done
clear
C)
GH
done
clear
D)
TSH
done
clear
View Answer play_arrow
question_answer 129) Which of the following substances can cure Parkinsons disease?
A)
GABA
done
clear
B)
Acetylcholine
done
clear
C)
Dopamine
done
clear
D)
Glutamic acid
done
clear
View Answer play_arrow
question_answer 130) Movement of tongue muscle is controlled by
A)
facial nerve
done
clear
B)
trigeminal nerve
done
clear
C)
hypoglossal nerve
done
clear
D)
vagus nerve
done
clear
View Answer play_arrow
question_answer 131) Which function will be lost due to damage of occipital lobe?
A)
Hearing
done
clear
B)
Speech
done
clear
C)
Vision
done
clear
D)
Memory
done
clear
View Answer play_arrow
question_answer 132) Meissners corpuscles occur in
A)
brain
done
clear
B)
nerve cells
done
clear
C)
skin
done
clear
D)
tongue
done
clear
View Answer play_arrow
question_answer 133) Osteomalacia is a deficiency disease of
A)
infants due to protein energy malnutrition
done
clear
B)
adults due to protein energy malnutrition
done
clear
C)
adults due to vitamin-D deficiency
done
clear
D)
infants due to vitamin-K deficiency
done
clear
View Answer play_arrow
question_answer 134) The gene of sickle cell anaemia is inherited by
A)
blood cells
done
clear
B)
bone cells
done
clear
C)
sex chromosomes
done
clear
D)
autosomes
done
clear
View Answer play_arrow
question_answer 135) Ptyalin is inactivated by a component of gastric juice known as
A)
pepsin
done
clear
B)
mucus
done
clear
C)
renin
done
clear
D)
HCl
done
clear
View Answer play_arrow
question_answer 136) Which one of the following human cells do not contain mitochondria?
A)
Nerve cell
done
clear
B)
Red blood cell
done
clear
C)
Liver cell
done
clear
D)
White blood cell
done
clear
View Answer play_arrow
question_answer 137) In which stage of the first meiotic division two sister chromatids are formed?
A)
Leptotene
done
clear
B)
Zygotene
done
clear
C)
Pachytene
done
clear
D)
Diplotene
done
clear
View Answer play_arrow
question_answer 138) Which one of the following triplet codon is a chain termination codon?
A)
UGU
done
clear
B)
AAU
done
clear
C)
UUG
done
clear
D)
UAG
done
clear
View Answer play_arrow
question_answer 139) How many pairs of contrasting characters in pea pod were chosen by Mendel?
A)
3
done
clear
B)
5
done
clear
C)
7
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 140) If a cross between two individuals produces offsprings with 50% dominant character and 50% recessive character the genotype of parents are
A)
\[Aa\times Aa\]
done
clear
B)
\[Aa\times aa\]
done
clear
C)
\[AA\times aa\]
done
clear
D)
\[AA\times Aa\]
done
clear
View Answer play_arrow
question_answer 141) Structural lipids of cell membrane are
A)
simple lipid
done
clear
B)
chromolipids
done
clear
C)
steroid
done
clear
D)
phospholipids
done
clear
View Answer play_arrow
question_answer 142) Which one of the following is polysaccharide?
A)
Glycogen
done
clear
B)
Sucrose
done
clear
C)
Lactose
done
clear
D)
Maltose
done
clear
View Answer play_arrow
question_answer 143) What will be the codons in mRNA if the DNA codes are ATG-CAG?
A)
TAC-GTC
done
clear
B)
UAC-GUC
done
clear
C)
UCA-TUA
done
clear
D)
TCA-GTC
done
clear
View Answer play_arrow
question_answer 144) Which of the following species is restricted to a specific area?
A)
Sibling species
done
clear
B)
Allopatric species
done
clear
C)
Sympatric species
done
clear
D)
Endemic species
done
clear
View Answer play_arrow
question_answer 145) Which of the following is not correctly matched?
A)
Sycon ? Canal system
done
clear
B)
Star fish ? Radial symmetry
done
clear
C)
Ascaris ? Flame cell
done
clear
D)
Prawn ? Haemocoel
done
clear
View Answer play_arrow
question_answer 146) Which one of the following animal phyla does not possess a coelom?
A)
Platyhelminthes
done
clear
B)
Annelida
done
clear
C)
Mollusca
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 147) Cardiac muscles are
A)
striated and voluntary
done
clear
B)
striated and involuntary
done
clear
C)
smooth and voluntary
done
clear
D)
smooth and involuntary
done
clear
View Answer play_arrow
question_answer 148) Which one of the following immunoglobulins is found as pentamer?
A)
IgG
done
clear
B)
IgM
done
clear
C)
IgA
done
clear
D)
IgE
done
clear
View Answer play_arrow
question_answer 149) Which one of the following cells is not a phagocytic cell?
A)
Macrophage
done
clear
B)
Monocyte
done
clear
C)
Neutrophil
done
clear
D)
Basophil
done
clear
View Answer play_arrow
question_answer 150) Which one of the following is the most primitive ancestor of man?
A)
Homo habilis
done
clear
B)
Australopithecus
done
clear
C)
Ramapithecus punjabicus
done
clear
D)
Homo neanderthalensis
done
clear
View Answer play_arrow
question_answer 151) A female Anopheles mosquito can be recognized by
A)
proboscis and palpi are long and more or less of equal length
done
clear
B)
proboscis long and palpi short
done
clear
C)
proboscis short and palpi long
done
clear
D)
Both proboscis and palpi are short
done
clear
View Answer play_arrow
question_answer 152) The anterior V- spot in microfilaria of Wuchereria represents
A)
nerve ring
done
clear
B)
cervical papilla
done
clear
C)
excretory system
done
clear
D)
reproductive system
done
clear
View Answer play_arrow
question_answer 153) In a population, unrestricted reproductive capacity is called
A)
biotic potential
done
clear
B)
fertility
done
clear
C)
carrying capacity
done
clear
D)
birthrate
done
clear
View Answer play_arrow
question_answer 154) When the two ecosystems overlap each other, the area is called
A)
habitat
done
clear
B)
niche
done
clear
C)
ecotone
done
clear
D)
ecotype
done
clear
View Answer play_arrow
question_answer 155) Pyramid of energy in ecosystems is
A)
always upright
done
clear
B)
always inverted
done
clear
C)
mostly upright
done
clear
D)
mostly inverted
done
clear
View Answer play_arrow
question_answer 156) Which one of the following is mainly responsible for green houses effect?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[~{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 157) Which one of the following is an exotic carp species?
A)
Barbus stigma
done
clear
B)
Cyprinus carpio
done
clear
C)
Labeo bata
done
clear
D)
Cirrhinus mrigala
done
clear
View Answer play_arrow
question_answer 158) Which of the following two hormones are essential for induced breeding of fishes?
A)
TSH and ACTH
done
clear
B)
Oestrogen and progesterone
done
clear
C)
FSH and LH
done
clear
D)
Vasopressin and oxytocin
done
clear
View Answer play_arrow
question_answer 159) Which stage of malarial parasite is infective toman?
A)
Gametocyte
done
clear
B)
Merozoite
done
clear
C)
Cryptomerozoite
done
clear
D)
Sporozoite
done
clear
View Answer play_arrow
question_answer 160) The scientific name of the moth which produce tasar is
A)
Bombyxmori
done
clear
B)
Antherae mylitta
done
clear
C)
Antherae assamensis
done
clear
D)
Philosomia ricini
done
clear
View Answer play_arrow