Answer:
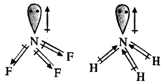
Both \[N{{F}_{3}}\] and \[N{{H}_{3}}\]
molecules have pyramidal geometries and their bond angles are also quite close
(102° and 107° respectively). But the dipole moment of \[N{{H}_{3}}\] is 1.49D
while that of \[N{{F}_{3}}\] is only 0.24D. This is mainly due to the reason
that the dipole moments of N-F bonds in \[N{{F}_{3}}\] molecule oppose the
dipole moment because of lone pair while in \[N{{H}_{3}}\] molecule, the dipole
moments of N-H bonds and lone pair reinforce. Thus, ammonia has more dipole
moment than \[N{{F}_{3}}\]. Because of the same reason, \[N{{F}_{3}}\] cannot
act as Lewis base just like \[N{{H}_{3}}\].
You need to login to perform this action.
You will be redirected in
3 sec
