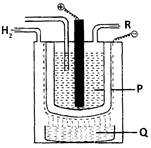
 When 'P' undergoes electrolysis 'Q' and 'R' are formed. 'Q' can be used in preparing soaps and detergents. 'R' can be used in the preparation of PVC, CFC and in swimming pools. What are P, Q and R?
When 'P' undergoes electrolysis 'Q' and 'R' are formed. 'Q' can be used in preparing soaps and detergents. 'R' can be used in the preparation of PVC, CFC and in swimming pools. What are P, Q and R?
A)
P
Q
R
\[NaHC{{O}_{3}}\]
\[N{{a}_{2}}C{{O}_{3}}C{{O}_{2}}\]
\[NaOH\]
B)
P
Q
R
\[NaOH\]
\[C{{l}_{2}}\]
\[NaCl\]
C)
P
Q
R
\[NaCl\]
\[NaOH\]
\[C{{l}_{2}}\]
D)
P
Q
R
\[N{{a}_{2}}C{{O}_{3}}\]
\[{{H}_{2}}O\]
\[C{{O}_{2}}\]
Correct Answer: C
Solution :
P is \[NaCl\] (Brine) which undergoes electrolysis and gives \[\text{NaOH}\](Q) at the cathode and \[C{{l}_{2}}(R)\] at the anode.You need to login to perform this action.
You will be redirected in
3 sec
