A) 1.20
B) 2.45
C) 3.55
D) 5.95
Correct Answer: C
Solution :
When an extra electron is added in nitrogen atom, added electron will be screened by five electrons in second orbit \[(2{{s}^{2}}2{{p}^{3}})\] and two electrons in first orbit \[(1{{s}^{2}}).\]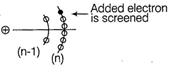 Now screening constant, \[\sigma =5\times 0.35\,\]in nth Orbit \[+\,2\times 0.85\]in \[(n-1)\,th\]orbit \[=1.75+1.70=3.45\] \[\therefore \] Effective nuclear charge \[=7-3.45=3.55\]
Now screening constant, \[\sigma =5\times 0.35\,\]in nth Orbit \[+\,2\times 0.85\]in \[(n-1)\,th\]orbit \[=1.75+1.70=3.45\] \[\therefore \] Effective nuclear charge \[=7-3.45=3.55\]
You need to login to perform this action.
You will be redirected in
3 sec
