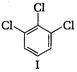
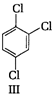
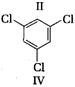
A) I < III < IV < II
B) II = IV < III < I
C) II = I < IV < III
D) III < I < II < IV
Correct Answer: B
Solution :
(II) and (IV) are symmetrical and thus, resultant dipole moment is zero. In (I) bond moment are towards the same direction but in (III) there is net dipole at the \[{{C}_{2}}\] position. Thus, the dipole moment of (I) is maximum.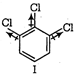
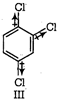 Thus, the correct order is II = IV < m < I
Thus, the correct order is II = IV < m < I
You need to login to perform this action.
You will be redirected in
3 sec
