A) \[[Xe]4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\]
B) \[[Xe]4{{f}^{8}}6{{s}^{2}}\]
C) \[[Xe]4{{f}^{9}}6{{s}^{1}}\]
D) \[[Xe]4{{f}^{10}}\]
Correct Answer: A
Solution :
\[Gd.(64)=[Xe]4{{f}^{7}}5{{d}^{1}}\]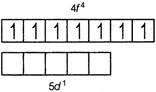 All electrons are unpaired, hence greater stability
All electrons are unpaired, hence greater stability
You need to login to perform this action.
You will be redirected in
3 sec
