A) square planar, 5
B) distorted tetrahedral, 4
C) trigonal bipyramidal, 5
D) trigonal pyramidal, 4
Correct Answer: C
Solution :
\[s{{p}^{3}}d\] hybridisation presents in\[PC{{l}_{5}}\]hence itsstructure is trigonal bipyramidal. It has\[5\sigma \]bond.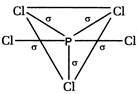
You need to login to perform this action.
You will be redirected in
3 sec
