A) Ethers
B) Alcohols
C) Carbonyl compounds
D) Carboxylic acids
Correct Answer: C
Solution :
The order of dipole moments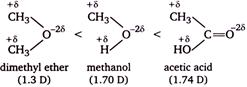
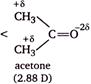 Methanol has higher dipole moment than dimethyl ether because oxygen of \[O-H\] bond can withdraw electrons from H atom more easily as compare to oxygen of \[O-C\] bond, thus becomes more polar. Acetic acid and acetone has higher dipole moment than methanol, because both has a \[s{{p}^{2}}-\]hybridized oxygen atom
Methanol has higher dipole moment than dimethyl ether because oxygen of \[O-H\] bond can withdraw electrons from H atom more easily as compare to oxygen of \[O-C\] bond, thus becomes more polar. Acetic acid and acetone has higher dipole moment than methanol, because both has a \[s{{p}^{2}}-\]hybridized oxygen atom You need to login to perform this action.
You will be redirected in
3 sec
