Answer:
(b) Electronic
configuration of carbon (C) = 2, 4 when it forms four covalent bonds by sharing
its four valence electrons with hydrogen, it forms ![]() molecule
like this
molecule
like this
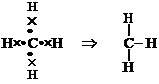 Now,
electronic configuration of C in
Now,
electronic configuration of C in ![]() Atomic
number of Ne is 10. Its electronic configuration is
Atomic
number of Ne is 10. Its electronic configuration is ![]() Therefore,
after the formation of four bonds, carbon attains the electronic configuration of
neon.
Therefore,
after the formation of four bonds, carbon attains the electronic configuration of
neon.
You need to login to perform this action.
You will be redirected in
3 sec
