Answer:
(b) It is an exothermic reaction because
heat is given out and the resulting compound is![]() which
is basic in nature so, the pH of the resulting solution will be more than seven.
which
is basic in nature so, the pH of the resulting solution will be more than seven.
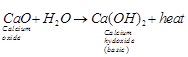
![]() turns
red litmus solution to blue. So, its pH value is greater than seven.
turns
red litmus solution to blue. So, its pH value is greater than seven.
You need to login to perform this action.
You will be redirected in
3 sec
