Answer:
For metals, low in the reactivity
series like mercury (Hg), the following steps are involved
(i) Roasting (heating the ore strongly
in the presence of excess of air) Metal sulphide is converted into metal oxide.
![]() (by heating in air)
(by heating in air)
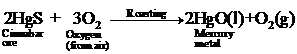 (ii) Reduction Metal oxide is
then reduced to metal by heating,
(ii) Reduction Metal oxide is
then reduced to metal by heating,
![]()
![]() (iii) Refining of metal.
For metals, medium in reactivity
series like zinc (Zn), the following steps are involved
(i) Roasting (for sulphide ores)
The sulphide ore is converted into metal oxide by heating the sulphide are
strongly in the presence of air.
(iii) Refining of metal.
For metals, medium in reactivity
series like zinc (Zn), the following steps are involved
(i) Roasting (for sulphide ores)
The sulphide ore is converted into metal oxide by heating the sulphide are
strongly in the presence of air.
![]() (ii) Reduction Metal oxides are
reduced to metals by using a suitable reducing agent. This can be done by
either of two.
(a) Reduction by heating with
carbon (smelting)
(ii) Reduction Metal oxides are
reduced to metals by using a suitable reducing agent. This can be done by
either of two.
(a) Reduction by heating with
carbon (smelting)
![]() (b) Reduction by heating with
aluminium
(b) Reduction by heating with
aluminium
![]()
You need to login to perform this action.
You will be redirected in
3 sec
