Answer:
(a) (i)![]() (ii)
(ii) ![]() (iii) Electrolytic refining
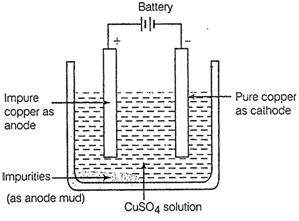
1.A thick block of the impure
metal is made anode (+ ve).
2.A thin strip of pure metal is
made cathode (- ve).
3.
(iii) Electrolytic refining
1.A thick block of the impure
metal is made anode (+ ve).
2.A thin strip of pure metal is
made cathode (- ve).
3.![]() solution is
taken as electrolyte.
At cathode
solution is
taken as electrolyte.
At cathode ![]() At anode
At anode ![]() (b) The diagram showing the
electrolytic refining of copper.
(b) The diagram showing the
electrolytic refining of copper.
You need to login to perform this action.
You will be redirected in
3 sec
