Answer:
Since, the element 'X'
of group 15 exists as a diatomic molecule and combines with hydrogen at 773 K
in presence of a catalyst to form ammonia which has a characteristic smell,
therefore, the element 'X' is nitrogen (N).
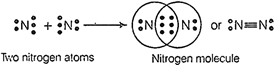
![]() (a) The atomic
number of nitrogen is 7. So, its electronic configuration is 2, 5. Thus, it has
five valence electrons.
(b) Nitrogen
has 5 valence electrons. Therefore, it needs 3 more electrons to complete its octet.
To do so, it shares three of its electrons with three electrons of the other
nitrogen atom to form a diatomic molecule of N2 gas.
Thus, three covalent
bonds are formed between two nitrogen atoms and each nitrogen atom is left
with one lone pair of electrons.
(a) The atomic
number of nitrogen is 7. So, its electronic configuration is 2, 5. Thus, it has
five valence electrons.
(b) Nitrogen
has 5 valence electrons. Therefore, it needs 3 more electrons to complete its octet.
To do so, it shares three of its electrons with three electrons of the other
nitrogen atom to form a diatomic molecule of N2 gas.
Thus, three covalent
bonds are formed between two nitrogen atoms and each nitrogen atom is left
with one lone pair of electrons.
 (c) Electron
dot structure for ammonia is as follows
(c) Electron
dot structure for ammonia is as follows
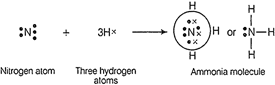 In NH3
molecule, there are three N?H single covalent bonds and one lone pair of electrons
on the nitrogen atom.
In NH3
molecule, there are three N?H single covalent bonds and one lone pair of electrons
on the nitrogen atom.
You need to login to perform this action.
You will be redirected in
3 sec
