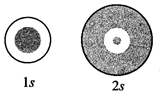
 The density of dots in a region
represents the probability density of finding electrons in the region.
On the basis of above diagram
which of the following statements is incorrect?
(a) 1s and 2s orbitals are
spherical in shape
(b) The probability of finding
the electron is maximum near the nucleus
(c) The probability of finding
the electron at a given distance is equal in all directions
(d) The probability density of
electrons for 2s orbital decreases uniformly as distance from the nucleus Increases
The density of dots in a region
represents the probability density of finding electrons in the region.
On the basis of above diagram
which of the following statements is incorrect?
(a) 1s and 2s orbitals are
spherical in shape
(b) The probability of finding
the electron is maximum near the nucleus
(c) The probability of finding
the electron at a given distance is equal in all directions
(d) The probability density of
electrons for 2s orbital decreases uniformly as distance from the nucleus Increases
Answer:
(d) Probability density
of 2s electrons does not decrease uniformly as distance from nucleus increases.
You need to login to perform this action.
You will be redirected in
3 sec
