Answer:
![]()
![]()
![]()
![]() Volume of sodium
target = A x d = 10-4 x 10-3 = 10-7 m3
We know that 6 x 1026 atoms of
Na weighs = 23 kg
Volume of sodium
target = A x d = 10-4 x 10-3 = 10-7 m3
We know that 6 x 1026 atoms of
Na weighs = 23 kg ![]() Volume of 6 x 106
Na atoms
Volume of 6 x 106
Na atoms ![]() Volume occupied by one Na atom
Volume occupied by one Na atom
![]() No.
of Na atoms in the target
No.
of Na atoms in the target
![]() Let n be the number of photons falling per second on the
target.
Energy
of each photon
Let n be the number of photons falling per second on the
target.
Energy
of each photon ![]() Total energy falling per sec on target
Total energy falling per sec on target ![]()
![]() Let
P be the probability of emission per atom per photon. The number of
photoelectrons emitted per second
Let
P be the probability of emission per atom per photon. The number of
photoelectrons emitted per second
![]() As pr question,
As pr question, ![]() Current ,
Current , ![]()
![]()
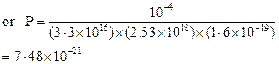 Thus the probability of
emission by a single photon on a single atom is very much less than 1. It is
due to this reason, the absorption of two photons by an atom is negligible.
Thus the probability of
emission by a single photon on a single atom is very much less than 1. It is
due to this reason, the absorption of two photons by an atom is negligible.
You need to login to perform this action.
You will be redirected in
3 sec
