Terminology Of Co-ordination Compounds
Category : JEE Main & Advanced
(1) Central metal atom or ion : A complex ion contains a metal atom or ion known as the central metal atom or ion. It is sometimes also called a nuclear atom.
(2) Complex ion : It is an electrically charged radical which is formed by the combination of a simple cation with one or more neutral molecules or simple anions or in some cases positive groups also.
(3) Ligands : Neutral molecules or ions that attach to central metal ion are called ligands. The donor atom associated with the ligands supplies lone pair of electrons to the central metal atom (forming dative bond) may be one or two more. Monodentate (one donor atom), bidentate (two donor atom), tridentate (three donor atom) etc.
Monodentate Ligands (with one donor site)
Anionic Ligands (Negative legands)
| Formula | Name | Formula | Name |
| \[{{X}^{-}}\] | Halo | \[O_{2}^{2-}\] | Peroxo |
| \[:O{{H}^{-}}\] | Hydroxo | \[C{{H}_{3}}CO{{O}^{-}}\] | Acetato |
| \[C{{N}^{-}}\] | Cyano | \[NO_{3}^{-}\] | Nitrato |
| \[{{O}^{2-}}\] | Oxo | \[{{S}_{2}}O_{3}^{2-}\] | Thiosulphato |
| \[NH_{2}^{-}\] | Amido | \[NO_{2}^{-}\] | Nitrito |
| \[{{S}^{2-}}\] | Sulphido | \[CO_{3}^{2-}\] | Carbonato |
| \[CN{{S}^{-}}\] | Thiocyanato | \[SO_{4}^{2-}\] | Sulphato |
Neutral Ligands
| Formula | Name | Formula | Name |
| CO | Carbonyl | \[:N{{H}_{3}}\] | Amminato |
| \[P{{H}_{3}}\] | Phosphine | \[{{H}_{2}}O\] | Aqua |
| NO | Nitrosyl | \[{{C}_{5}}{{H}_{5}}N:\] | Pyridine (py) |
Cationic Ligand (Positive)
| Formula | Name | Formula | Name |
| \[NO_{2}^{+}\] | Nitronium | \[N{{O}^{+}}\] | Nitrosonium |
| \[{{H}_{2}}NNH_{3}^{+}\] | Hydrazinium |
Polydentate ligands (with two or more donor site)
Bidentate (Two donor sites)
| Formula | Name | Formula | Name |
| \[{{H}_{2}}NC{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\] | Ethylenediamine (en) | \[\underset{\,Me\,-C=NOH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{Me-C=N{{O}^{-}}}}\,}}\,\] | Dimeth-ylglyoxi-meto (dmg) |
| \[\overset{-}{\mathop{O}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{O}^{-}}\] | Oxalato (ox) | \[N{{H}_{2}}-C{{H}_{2}}-CO{{O}^{-}}\] | Glyci-nate ion (gly) |
| Formula | Name | |
| Tridentate | \[{{H}_{2}}\overset{\bullet \bullet }{\mathop{N}}\,{{(C{{H}_{2}})}_{2}}-\overset{\bullet \bullet }{\mathop{N}}\,H-{{(C{{H}_{2}})}_{2}}\overset{\bullet \bullet }{\mathop{N}}\,{{H}_{2}}\,\] | Diethylenetriami-nediami-ne (dien) |
| Tetradentate | \[{{H}_{2}}\overset{\bullet \bullet }{\mathop{N}}\,{{(C{{H}_{2}})}_{2}}-\overset{\bullet \bullet }{\mathop{N}}\,H-{{(C{{H}_{2}})}_{2}}\overset{\bullet \bullet }{\mathop{N}}\,H{{(C{{H}_{2}})}_{2}}\overset{\bullet \bullet }{\mathop{N}}\,{{H}_{2}}\,\] | Triethyl-enetetra-mine (trien) |
| Hexadentate | Ethylen-ediamine tetra -acetic acid (EDTA)4? |
Chelating Ligand : When polydentate ligands bind to the central metal ion they form a ring called chelate and the ligand is referred as chelating ligand. Ambidentate ligands : A ligand which possesses two donor atom but in forming complex it utilizes only one atom depending upon the condition and type of complex.
\[N{{O}_{2}}\] (nitro) , ONO (nitrito), CN (cyano), NC (isocyano), SCN (thiocyanide), NCS (isothiocyanide)
\[\pi -\]acid ligand : Ligands which are capable of accepting an appreciable amount of \[\pi -\] \[{{e}^{-}}\] density from the metal atom into emptying \[\pi \] or \[{{\pi }^{*}}\] orbital or their own called \[\pi -\] acceptor or \[\pi -\] acid ligands eg. CO.
(4) Co-ordination Sphere : Ligand with central metal ion is kept in square bracket [ ] retains its identity in the same form is called co-ordination sphere (non-ionisable)
(5) Co-ordination Number : Number of monodentate ligands attached to central atom/ion are called coordination number of the central metal atom/ion.
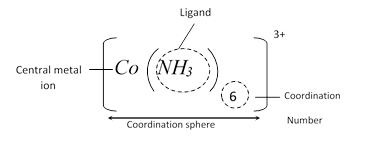
(6) Ionisation Sphere : The part present out side of the square bracket is called ionization sphere (ionisable).
You need to login to perform this action.
You will be redirected in
3 sec
