Isochoric or Isometric Process
Category : JEE Main & Advanced
When a thermodynamic process undergoes a physical change in such a way that its volume remains constant, then the change is known as isochoric process.
(1) Equation of state : In this process P and T changes but V = constant. Hence Gay-Lussac?s law is obeyed in this process i.e. \[P\propto T\Rightarrow \frac{{{P}_{1}}}{{{T}_{1}}}=\frac{{{P}_{2}}}{{{T}_{2}}}=\] constant
(2) Indicator diagram : Graph 1 and 2 represent isometric increase in pressure at volume \[{{V}_{1}}\] and isometric decrease in pressure at volume \[{{V}_{2}}\] respectively and slope of indicator diagram\[\frac{dP}{dV}=\infty \]
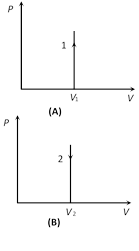
(i) Isometric heating
(a) Pressure \[\xrightarrow{\,}\] increases
(b) Temperature \[\xrightarrow{\,}\] increases
(c) \[\Delta Q\] \[\xrightarrow{\,}\]positive
(d) \[\Delta U\] \[\xrightarrow{\,}\]positive
(ii) Isometric cooling
(a) Pressure \[\xrightarrow{\,}\] decreases
(b) Temperature \[\xrightarrow{\,}\] decreases
(c) \[\Delta Q\] \[\xrightarrow{\,}\]negative
(d) \[\Delta U\]\[\xrightarrow{\,}\]negative
(3) Specific heat : Specific heat of gas during isochoric process \[{{C}_{V}}=\frac{f}{2}R\]
(4) Bulk modulus of elasticity : \[K=\frac{\Delta P}{\frac{-\Delta V}{V}}=\frac{\Delta P}{0}=\infty \]
(5) Work done in isochoric process \[\Delta W=P\Delta V=P[{{V}_{f}}-{{V}_{i}}]=0\] [As V = constant]
(6) FLOT in isochoric process : From \[\Delta Q=\Delta U+\Delta W\]
\[\because \] \[\Delta W=0\Rightarrow {{(\Delta Q)}_{V}}=\Delta U=\mu {{C}_{V}}\,\Delta T=\mu \frac{R}{\gamma -1}\Delta T=\frac{{{P}_{f}}{{V}_{f}}-{{P}_{i}}{{V}_{i}}}{\gamma -1}\]
You need to login to perform this action.
You will be redirected in
3 sec
