Acids, Bases, Salts and Metals
Category : UPSC
ACIDS, BASES, SALTS AND METALS
ACIDS, BASES AND SALTS
Some naturally occurring substances that contain acids are given in the following table:
|
Substances |
Acid present |
|
Orange, lemon |
Citric acid, ascorbic acid (vitamin c) |
|
Apple |
Malic acid |
|
Tamarind |
Tartaric acid |
|
Vinegar |
Acetic acid |
|
Curd |
Lactic acid |
|
Tomato |
Oxalic acid |
|
Gastric juice |
Hydrochloric acid |
|
Tea |
Tannic acid |
|
Red ants |
Formic acid |
Swedish scientist Svante Arrhenius in the late nineteenth century first attempted to explain the behaviour of acids and bases from their chemical structure.
CONCEPTS OF ACIDS AND BASES
Arrhenius Concept
According to Arrhenius, an acid is a compound that releases \[{{H}^{+}}\]ions in water; and a base is a compound that releases \[O{{H}^{-}}\] ions in water.
Bronsted-Lowry Concept
In 1923 J.N. Bronsted and J.M. Lowry independently proposed a broader concept of acids and bases. According to this concept,
Conjugate Acid-Base pairs
An important concept that emanates from Bronsted-Lowry concept is conjugate acid-base pairs. In an acid-base reaction the acid (HA) gives up its proton (\[{{H}^{+}}\]) and produces a new base (\[{{A}^{-}}\]).
Classes of Bronsted Acids and Bases
Bronsted acids can be classified as per its capacity to furnish protons as follows:
Strength of Bronsted Acids and Bases
The strength of a Bronsted acid depends upon its tendency to donate a proton. The strength of a Bronsted base depends on its ability to accept a proton.
For example, HCl is nearly 100% ionised in water. Its reaction with water can be depicted by the equation:
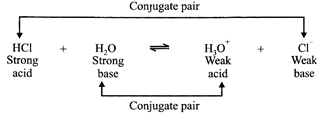
The above reaction has proceeded almost completely to the right; that indicates that HCl has a strong tendency to lose a proton. Also, the base \[{{H}_{2}}O\] has a strong ability to accept a proton. The overall situation is that the acid and base on the left are each stronger than the conjugate acid and conjugate base on the right. That is why the equilibrium is displaced to the right. Thus it may be stated that:
Strong Acids: \[HCl,HBr,Hl,HN{{O}_{3}},{{H}_{2}}S{{O}_{4}},HCI{{O}_{3}},HCI{{O}_{4}}\]
Strong Bases: Alkali metal hydroxides, \[Ba{{(OH)}_{2}},Sr{{(OH)}_{2}},Ca{{(OH)}_{2}},Mg{{(OH)}_{2}}\]
Lewis Concept of Acids and Bases
In the early 1930s, G.N. Lewis gave an even a more general model of acids and bases. According to Lewis theory:
Lewis visualized an acid and base as sharing the electron pair provided by the base. As a result a covalent bond (or coordinate bond) between the Lewis acid and the Lewis base is formed. The resulting combination is called a Complex.
pH SCALE-THE MEASURE OF ACIDITY
The concentrations of \[{{H}^{+}}\] and \[O{{H}^{-}}\] ions in aqueous solutions are frequently very small and hence not convenient to work with.
\[pH=-\log [{{H}_{3}}{{O}^{+}}]\] or \[pH=-\log [H]\]
\[pOH=\log [O{{H}^{-}}]\]
\[p{{H}^{+}}pOH=14.00\]
pH in Humans and Animals
Most of the biochemical reactions taking place in our body are m a narrow \[pH\] range of 7.0 to 7.8. Even a small change in \[pH\]bumpers the processes. Any condition in which blood \[pH\]drops below 7.35 is known as acidosis, if \[pH\] rises above 7.45-then it is called alkalosis.
Acid Rain
When the \[pH\] of rain water goes below 5.6, it is called acid rain.
Acid rain is a major environmental disaster.
pH in Plants
Soils need to be of optimum\[pH\]for plants to have an adequate growth .It should be neither highly alkaline nor highly acidic.
In digestive system
pH plays an important part in the digestion of food. Our stomach produces hydrochloric acid (formic acid) which helps in digestion of food. When we eat spicy food, stomach produces too much of acid which causes ‘acidity’ i.e. irritation and sometimes pain too. In order to get cured from this we use ‘antacids’ which are bases like ‘milk of magnesia’ (suspension of magnesium hydroxide in water).
Self-defence of Animals and Plants
Bee sting causes severe pain and burning sensation. It is due to the presence of methanoic acid (formic acid) in it. Use of a mild base like baking soda can provides relief from pain. Some plants like ‘nettle plant’ have fine stinging hair which inject methanoic acid into the body of any animal or human being that comes in its contact.
BUFFER SOLUTIONS
Generally pH of an aqueous solution decreases on addition of a small amount of HCl because of the increase in the concentration of \[{{H}^{+}}\]ions. On the other hand, if a small amount of NaOH is added, the pH of the solution increases. However, there are some solutions which resist the change in pH on addition of small amount of strong acid or alkali. Such solutions are called buffer solutions. For example, solution of ammonium acetate, blood, a equimolar mixture of \[N{{H}_{4}}OH+N{{H}_{4}}Cl,C{{H}_{3}}COOH+C{{H}_{3}}COONa,\]etc.
Types of Buffer Solution
A salt is an ionic compound which dissociates to yield a positive ion other than hydrogen ion [\[{{H}^{+}}\]] and a negative ion other than hydroxide ion [\[O{{H}^{-}}\]]
Example:
\[NaCl\to N{{a}^{+}}+C{{l}^{-}}\](fused/ Aq. soln)
Classification of Salts
Examples:
\[Cu(OH)N{{O}_{3}}\]- Basic copper nitrate.
Example:
\[FeS{{O}_{4}}{{(N{{H}_{4}})}_{2}}S{{O}_{4}}6{{H}_{2}}O\](Mohr’s salt) is a mixture of \[FeS{{O}_{4}}\] (Simple salt) and \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\](Simple salt)
Examples: Sodium potassium sulphate \[NaKS{{O}_{4}}\] (two basic radicals,\[N{{a}^{+}}\],\[{{K}^{+}}\])
SOME COMMON USEFUL SALTS
A large number of salts are useful for our homes and industry for various purposes. Some are discussed below:
Baking Soda
Chemically baking soda is sodium hydrogen carbonate; \[NaHC{{O}_{3}}\].
\[2NaHC{{O}_{3}}\xrightarrow[{}]{heat}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\uparrow \]
Uses:
\[2NaHC{{O}_{3}}\xrightarrow[{}]{heat}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\uparrow \]
Washing Soda
Uses
Plaster of Paris
Also called POP, chemically, it is \[2CaS{{O}_{4}}.{{H}_{2}}O\]or \[CaS{{O}_{4}}.1/2{{H}_{2}}O\] (calcium sulphate hemi hydrate)
Uses
Bleaching Powder
Bleaching is a process of removing colour from a cloth to make it whiter. Bleaching powder has been used for this purpose since long. Chemically, it is calcium oxy chloride\[CaOC{{l}_{2}}\].
Uses:
Sodium Hydroxide
Also known as Caustic Soda, chemically it is NaOH. Industrial methods of its production are:
Handy Facts
Some properties of NaOH are:
NaOH (caustic soda) is a white translucent solid available in pellets, flakes and granules as 50% saturated solution.
In solution when exposed to air reacts with atmospheric \[C{{O}_{2}}\]and from a crust of \[N{{a}_{2}}C{{O}_{3}}\]at the surface.
Uses:
Sodium Carbonate
Also called soda ash, its chemical formula is \[N{{a}_{2}}C{{O}_{3}}\]
It exists in various forms, namely anhydrous sodium carbonate \[N{{a}_{2}}C{{O}_{3}}\] (Soda-ash). Monohydrate \[N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O\] (crystal carbonate), heptahydrate. \[N{{a}_{2}}C{{O}_{3}}.7{{H}_{2}}O\] and decahydrate \[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\] (washing soda or sal soda)
Sodium carbonate is generally prepared now a days by ammonia soda or Solvay process. The ingredients of this process are readily available and inexpensive. These are Salt brine (NaCl) (from sea), ammonia (\[N{{H}_{3}}\]) and lime stone \[CaC{{O}_{3}}\](from mines). The process consists of many sections, \[CaC{{l}_{2}}\] is an important by-product obtained.
Uses:
It is used for the manufacture of glass, borax, soap and caustic soda (NaOH)
PROPERATIES, EXTRACTION AND USES OF METALS
Out of 118 chemical elements known till date, 103 has been well characterized in terms of their properties. The systematic classification of these 103 elements shows their numbers as:
COMPARISON OF METALS AND NON-METALS
Metals and non-metals differ both in physical and chemical properties.
The characteristic physical properties of metals and non-metals are listed in following table:
|
Serial No. |
Metals |
Non-metals |
|
1 |
Metals have luster. They reflect light from polished or freshly cut surface |
Non-metals do not have luster( exceptions - Diamond and Iodine ) |
|
2 |
Metals generally have high density. |
Non-metals generally have low density. |
|
3 |
They are good conductors of heat and electricity. |
They are usually bad conductors of heat (exception - carbon in the form of gas carbon and graphite ) |
|
4 |
Metals are malleable and ductile. They can be beaten into thin sheets and drawn into wires. |
Non-metals are not malleable and ductile. They can be crushed into powder. |
|
5 |
They have a three dimensional crystal structure with metallic bonds |
They have different types of structures with covalent and van-der-Waals bonds |
|
6 |
Metals are generally hard. |
Non- metals are generally soft (Exception: Diamond) |
|
7 |
Metals generally have 1 to 3 electrons in their outermost shell |
Non-metals generally have 4 to 8 in their outermost shell of the atom. |
|
8 |
They show valency 1 to 4. |
They show valency 1 to 7. |
|
9 |
They are electropositive in nature. |
They are electronegative in nature. j |
|
10 |
They generally form basic oxides. |
They generally form acidic oxides. |
|
11 |
They act as reducing agents. |
They act as oxidizing agents. |
|
12 |
Active metals react with cold and hot water. |
Non-metals usually do not react with cold or hot water. |
|
13 |
Active metals react with non- oxidizing acids to form their oxides or oxy acids. |
Solid non-metals react with oxidizing acids to form hydrogen gas. |
|
14 |
They react with non-metals under different conditions to form salts. |
They react with metals as well as non-metals under different conditions to form salts. |
CHEMICAL PROPERTIES OF METAL
The chemical reactions undergone by metals are furnished below:
Reaction with Oxygen
Most of the metals react with oxygen and form oxides. The reaction may take place without heating as in sodium, calcium or potassium, while some metals react with oxygen on heating to form oxides.
Reaction of Metals with Acids
Metals react with common acids like dilute HCl and dilute \[{{H}_{2}}S{{O}_{4}}\] with evolution of\[{{H}_{2}}\].
Reaction of Metals with Water
Many metals react with water to form hydroxides. Hydroxides are basic in nature. Sodium and potassium react with cold water.
\[Mg(s)+{{H}_{2}}O(l)\to Mg{{(OH)}_{2}}(aq)+{{H}_{2}}(g)\]
Reaction of Metals with Common Bases
Some metals like aluminium and zinc react with common bases.
\[Sn(s)+2NaOH(aq)+{{H}_{2}}O(l)\to N{{a}_{2}}Sn{{O}_{3}}\]
sodium stannate
\[Zn(s)+2NaOH(aq)\to N{{a}_{2}}Zn{{O}_{2}}\]
Sodium zincate
REACTIVITY SERIES
A metal that can lose electrons more easily in comparison to other metal is more electropositive and would be more active in nature then other metal. Such a metal when dipped in a solution of salt of a less active metal would displace it.
The arrangement of metals in the decreasing order of their activity is known as activity or reactivity series. It is also known as electrochemical series.
EXTRACTION OF METALS-METALLURGY
The process of extraction of metal from its ore is called metallurgy.
Examples
Examples of Ores
Some important ores and the metals present in these ores are listed in the following table:
|
Type of ore |
Metals |
|
Native Metals (Found in Free State) |
Gold (Au), silver (Ag) |
|
Oxide ores |
Iron (Haematite, \[F{{e}_{2}}{{O}_{3}}\]); Aluminum (Bauxite, \[A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O\]); Tin(Cassiterite, \[Sn{{O}_{2}}\]); Copper Cuprite, \[C{{u}_{2}}O\]); Zinc (Zincite, ZnO); Titanium (llmenite, \[FeTi{{O}_{3}}\], Rutile, \[Ti{{O}_{2}}\]) |
|
Sulphide ores |
Zinc (Zinc blende, ZnS); Lead (Galena, PbS); Copper (Copper glance, \[C{{u}_{2}}S\]); Silver (Silver glance or Argentite, \[A{{g}_{2}}S\]); Iron(Ironpyrites, \[Fe{{S}_{2}}\]) |
|
Carbonate ores |
Iron (Siferite, \[FeC{{O}_{3}}\]); Zinc (Calamine, \[ZnC{{O}_{3}}\]), Lead (Cerrusite, \[PbC{{O}_{3}}\]) |
|
Sulphate ores |
Lead(Anglesite, \[PbS{{O}_{4}}\]) |
|
Halide ores |
Silver (Horn silver, AgCl); Sodium (Common salt or Rock salt. NaCl); Aluminum (Cryolite, \[N{{a}_{3}}AI{{F}_{6}}\]) |
|
Silicate ores |
Zinc (Hemimorphite, \[2ZnO.Si{{O}_{2}}.{{H}_{2}}O\]) |
The method used to extract metals from the ore in which they are found depends on their reactivity.
Steps Involved in the Extraction of Metals from Ores
Metallurgical Steps
Few steps involved in extracting the metal as shown above are discussed below:
Concentrating the Ore
This means getting rid of as much of the unwanted rocky material (called gangue or matrix) as possible before the ore is converted into the metal.
Some methods are:
Froth floatation: The ore is first crushed and then treated with something which will bind to the particles of the metal compound that are required and make those particles hydrophobic. “Hydrophobic” stands for “water fearing”.
Magnetic separation: Magnetic ores like pyrolusite (\[Mn{{O}_{2}}\]) and chromite (\[FeO.C{{r}_{2}}{{O}_{3}}\]) are enriched by this method by making use of the difference in the magnetic properties of the ore and gangue particles.
Conversion of concentrated ore to oxide
It is easier to obtain a metal from its oxide form as compared to its sulphide, carbonate or any other form. Therefore, prior to reduction usually the metal is converted to its oxide form.
Following methods are used to convert the concentrated ore to its oxide form.
Calcination: It is a process in which the ore is heated strongly in absence of air. The ore is heated at a temperature well below its melting point.
This method is generally used for carbonate and hydrated ores.
Roasting: It is a process wherein the ore is heated either alone or with some other material in excess of air below the fusion point of the ore.
The differences between roasting and calcinations are given in the following table:
Reducing the metal compound to the metal
Carbon (as coke or charcoal) is cheap. It not only acts as a reducing agent, but it also acts as the fuel to provide heat for the process.
This is a common extraction process for the more reactive metals - for example, for aluminium and metals above it in the electrochemical series.
An advantage is that it can produce very pure metals.
Refining of metals
Refining of metals is done to obtain metals in very pure form by removing impurities present in it. Electrolytic refining is widely used method for this purpose.
ALLOYS
Alloys are metallic materials prepared by mixing two or more molten metals.
These are used for many purposes, such as construction, and are central to the transportation and electrical industries. Composition of some common alloys and their uses are given in the following table.
|
|
Alloy |
Composition |
Uses |
|
1. |
Brass |
Cu = 80%, Zn = 20% |
For making utensils and cartridges. |
|
2. |
Bronze |
Cu = 90%, Sn = 10% |
For making statues, medals, ships, coins and machines |
|
3. |
Solder |
Sn = 50%, Pb = 50% |
For joining metals, solding wire and electronic components etc. |
|
4. |
Duraluminum |
Al = 95.5%, Cu = 3%, |
Used in bodies of aircrafts, kitchen parts etc. ware and automobile |
|
|
|
Mn = 1.0%, Mg = 0.5% |
|
|
5. |
German Silver |
Cu = 60%, Zn = 20%, Ni = 20% |
For making utensils and ornaments |
|
6. |
Gun metal |
Cu = 90%, Sn = 10% |
For Gears and castings etc. |
|
7. |
Bell metal |
Cu = 80%, Sn = 20% |
For bells, gangs etc. |
|
8. |
Magnalium |
Al = 90%,Mg=10% |
For balance beams, light instruments. |
|
9. |
Type metal |
Pb = 82%, Sb = 15%, Sn = 3% |
For casting type |
|
10. |
Stainless steel |
Fe, Ni, Cr, C |
For utensils, cutlery etc. |
An amalgam is an alloy of mercury with one or more metals. Most of the metals form amalgams with mercury except iron and platinum. Amalgams of sodium and aluminium are good reducing agents. Amalgam of silver, tin, cadmium and copper have been utilized as dental fillings.
SOME IMPORTANT METALS AND THEIR USES
Iron (Fe)
Iron is the most abundant metal which occurs in the earth’s crust. The most commonly used iron ores are haematite, \[F{{e}_{2}}{{O}_{3}}\], and magnetite, \[F{{e}_{3}}{{O}_{4}}\].
Cast Iron:
The molten iron from the bottom of the furnace can be used as cast iron. However, it is very impure, containing about 4% of carbon. This carbon makes it very hard, but also very brittle. If you hit it hard, it tends to shatter rather than bend or dent.
Use: Cast iron is used for things like manhole covers, cast iron pipes, valves and pump bodies in the water industry, guttering and drainpipes, cylinder blocks in car engines,
Aga-type cookers, and very expensive and very heavy cookware.
Wrought iron
If all the carbon is removed from the iron to give high purity iron, it is known as wrought iron. Wrought iron is quite soft and easily worked and has little structural strength.
Use: It was once used to make decorative gates and railings, but these days mild steel is normally used instead.
Mild steel
Mild steel is iron containing up to about 0.25% of carbon. The presence of the carbon makes the steel stronger and harder than pure iron. The higher the percentage of carbon, the harder the steel becomes.
Use: Mild steel is used for lots of things - nails, wire, car bodies, ship building, girders and bridges amongst others.
High carbon steel
High carbon steel contains up to about 1.5% of carbon. The presence of the extra carbon makes it very hard, but it also makes it more brittle.
Use: Used for cutting tools and masonry nails (nails designed to be driven into concrete blocks or brickwork without bending). It tends to fracture rather than bend if you mistreat it.
Special steels
These are iron alloyed with other metals. Examples are given in the following table:
|
Variety |
Iron mixed with |
Special Properties |
Uses |
|
Painless steel |
chromium and nickel |
resists corrosion |
cutlery, cooking utensils, kitchen sinks, industrial equipment for food and drink processing |
|
titanium steel |
titanium |
withstands high temperatures |
gas turbines, spacecraft |
|
manganese steel |
manganese |
very hard |
rock-breaking machinery, some railway track (e.g. points), military helmets |
Handy Facts
The hardness and elasticity of steel can be controlled by heat treatment. The steel is heated to a temperature below redness. It is then cooled slowly. The process is called tempering of steel. It is used to bring the steel to a suitable state of hardness and elasticity. Hard steel can be softened by heating it to a high temperature and then allowing it to cool down slowly. This process is called annealing.
Steel produced in this way is known as quenched steel and the process of making such is known as quenching or hardening of steel
Some important compounds of iron:
Green Vitriol: Iron sulphate or ferrous sulphate is the chemical compound with the formula (\[FeS{{O}_{4}}\]).\[FeS{{O}_{4}}\]. \[7{{H}_{2}}O\]is called Green vitriol.
It is used as:
Mohr’s Salt: Ammonium iron sulphate, or Mohr’s Salt, is a double salt of iron sulphate and ammonium sulphate, with the formula\[{{[N{{H}_{4}}]}_{2}}[Fe]{{[S{{O}_{4}}]}_{2}}.6{{H}_{2}}O\]. It is used in the laboratory.
Copper (Cu)
Copper is malleable and ductile and is a good conductor of both heat and electricity. The important ores of copper is chalcopyrite, \[CuFe{{S}_{2}}\](also known as copper pyrites)
Copper has multifarious uses like:
Alloying produces a metal harder than either copper or zinc individually. Bronze is another copper alloy – this time with tin.
Some important compounds of Copper
Copper sulphate is the compound with the formula\[CuS{{O}_{4}}\]. This salt exists as a series of compounds that differ in their degree of hydration. The anhydrous form is a pale green or gray – white powder, whereas the penta hydrate (\[CuS{{O}_{4}}.5{{H}_{2}}O\]), the most commonly encountered salt, is bright blue.
Copper oxide or cupric oxide (CuO) is the higher oxide of copper. As a mineral, it is known as tenorite.
Uses:
Uses
Cuprous oxide is commonly used as a pigment, a fungicide, and an antifouling agent for marine paints.
Rectifier based on this material have been used industrially.
Aluminium
The usual aluminium ore is bauxite. Bauxite is essentially an impure aluminium oxide. The major impurities include iron oxides, silicon dioxide and titanium dioxide. Bauxite actually contains one of a variety of hydrated aluminium oxides some of which can be written as\[A{{l}_{2}}{{O}_{3}},x{{H}_{2}}O\].
Uses: Aluminium is used in aircraft making, container vehicle bodies, tube trains, in making saucepans, etc.
Science in Action
Anodizing of Aluminium: In anodising the aluminium is first etched with sodium hydroxide solution to remove the existing oxide layer, and then making the aluminium article the anode in electrolysis of dilute sulphuric acid. The oxygen given off at a film of oxide up to about 0.02 mm thick.
Zinc (Zn)
It is extracted from ores like zinc blende (ZnS).
Handy Facts
Important uses of some other metals are also listed below:
You need to login to perform this action.
You will be redirected in
3 sec
