Answer:
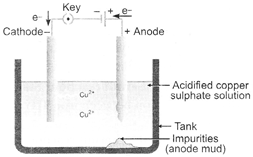
In electrolytic refining process,
the impure metal is made as anode and a thin strip of pure c metal is made as
cathode. A solution of the metal salt is made as an electrolyte. On passing the
current through the electrolyte, the pure metal from the anode dissolves into
the electrolyte. An equivalent amount of pure metal from the electrolyte is
deposited on the cathode. The soluble impurities go into the solution, whereas,
the insoluble impurities settle down at the bottom of the anode and are known
as anode mud.
At anode:
Cu ![]() Cu2++ 2e-
At
cathode: Cu2+ + 2e-
Cu2++ 2e-
At
cathode: Cu2+ + 2e-![]() Cu
Cu
You need to login to perform this action.
You will be redirected in
3 sec
