Answer:
(i) Elements which
contain 1 to 3 electrons in their outermost shell are metals.
Elements
containing 4 to 7 electrons in their valence shell are non-metals.
Electronic
configurations:
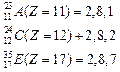
![]() Hence
A and C are metals whereas, B, D and E are non-metals.
(ii)
Type of bonds
(a)'A'
is metal and 'B' is non-metal, so the bond formed will be ionic.
A =
2, 8, 1, B = 2, 7
Hence
A and C are metals whereas, B, D and E are non-metals.
(ii)
Type of bonds
(a)'A'
is metal and 'B' is non-metal, so the bond formed will be ionic.
A =
2, 8, 1, B = 2, 7
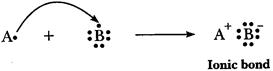 (b)
'A' is metal and 'E' is non-metal, so the bond formed is ionic.
A
=2, 8, 1 E=2, 8, 7
(b)
'A' is metal and 'E' is non-metal, so the bond formed is ionic.
A
=2, 8, 1 E=2, 8, 7
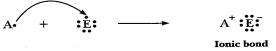 (c) 'C' is metal
and 'E' is non-metal, so the bond formed is ionic.
(c) 'C' is metal
and 'E' is non-metal, so the bond formed is ionic.
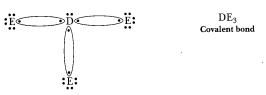
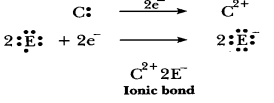 (d) 'D' is a
non-metal and E is also a non-metal, so the bond formed will be covalent.
D=2,
8, 5 E=2, 8, 7
(d) 'D' is a
non-metal and E is also a non-metal, so the bond formed will be covalent.
D=2,
8, 5 E=2, 8, 7
You need to login to perform this action.
You will be redirected in
3 sec
