Answer:
(i) Due to \[+I\]-effect of the \[C{{H}_{3}}C{{H}_{2}}\] groups, \[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]and \[{{({{C}_{2}}{{H}_{5}})}_{3}}N\] are stronger bases than \[N{{H}_{3}}\]. However, due to delocalization of the lone pair of electrons present on nitrogen over the benzene ring,\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\] is a weaker base than \[{{({{C}_{2}}{{H}_{5}})}_{2}}NH,\,\,{{({{C}_{2}}{{H}_{5}})}_{3}}N\]and \[N{{H}_{3}}\].
The relative basic strength of \[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\] and \[{{({{C}_{2}}{{H}_{5}})}_{3}}N\] depends upon the stabilization of their conjugate acids (formed as a result of accepting a proton from water) by a number of factors such as H- bonding, steric hindrance of the alkyl groups and \[+I\]-effect of the alkyl groups.
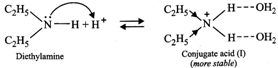
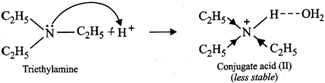 Now conjugate acid \[(II)\] is more stable due \[+I\]-effect of the three \[{{C}_{2}}{{H}_{5}}\] groups but less stable due to steric hindrance to H-bonding by three \[{{C}_{2}}{{H}_{5}}\] groups.
In contrast, conjugate acid \[(I)\] is less stable due to \[+I\]-effect of only two \[{{C}_{2}}{{H}_{5}}\] groups but more stable due to less steric hindrance to H-bonding due to two \[{{C}_{2}}{{H}_{5}}\] groups. Since stabilization due to steric hindrance to H-bonding predominates over \[+I\]-effect of the alkyl groups, therefore, conjugate acid \[(I)\] is more stable than conjugate acid \[(II)\] and hence \[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\] is more basic than \[{{({{C}_{2}}{{H}_{5}})}_{3}}N\] . Thus, the overall basic strength of the four amines increases in the order :\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}<N{{H}_{3}}<{{({{C}_{2}}{{H}_{5}})}_{3}}N<{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
(ii) In the gas phase, solvent effects are absent and hence the relative basicity of amines depends only on the \[+I\]-effect of the alkyl groups. Now the \[+I\]-effect of the \[{{C}_{2}}{{H}_{5}}\] group is more than that of \[C{{H}_{3}}\] group and the \[+I\]-effect increases as the number of alkyl groups increases. Therefore, overall basicity of the four amines increases in the order:\[C{{H}_{3}}N{{H}_{2}}<{{C}_{2}}{{H}_{5}}N{{H}_{2}}<{{({{C}_{2}}{{H}_{5}})}_{2}}\]\[NH<{{({{C}_{2}}{{H}_{5}})}_{3}}N\]
Now conjugate acid \[(II)\] is more stable due \[+I\]-effect of the three \[{{C}_{2}}{{H}_{5}}\] groups but less stable due to steric hindrance to H-bonding by three \[{{C}_{2}}{{H}_{5}}\] groups.
In contrast, conjugate acid \[(I)\] is less stable due to \[+I\]-effect of only two \[{{C}_{2}}{{H}_{5}}\] groups but more stable due to less steric hindrance to H-bonding due to two \[{{C}_{2}}{{H}_{5}}\] groups. Since stabilization due to steric hindrance to H-bonding predominates over \[+I\]-effect of the alkyl groups, therefore, conjugate acid \[(I)\] is more stable than conjugate acid \[(II)\] and hence \[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\] is more basic than \[{{({{C}_{2}}{{H}_{5}})}_{3}}N\] . Thus, the overall basic strength of the four amines increases in the order :\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}<N{{H}_{3}}<{{({{C}_{2}}{{H}_{5}})}_{3}}N<{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
(ii) In the gas phase, solvent effects are absent and hence the relative basicity of amines depends only on the \[+I\]-effect of the alkyl groups. Now the \[+I\]-effect of the \[{{C}_{2}}{{H}_{5}}\] group is more than that of \[C{{H}_{3}}\] group and the \[+I\]-effect increases as the number of alkyl groups increases. Therefore, overall basicity of the four amines increases in the order:\[C{{H}_{3}}N{{H}_{2}}<{{C}_{2}}{{H}_{5}}N{{H}_{2}}<{{({{C}_{2}}{{H}_{5}})}_{2}}\]\[NH<{{({{C}_{2}}{{H}_{5}})}_{3}}N\]
You need to login to perform this action.
You will be redirected in
3 sec
